当前位置:
X-MOL 学术
›
Biomed. Pharmacother.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cyclophosphamide enfeebles myocardial isometric contraction force via RIP1/RIP3/MLKL/TRPM7-mediated necroptosis
Biomedicine & Pharmacotherapy ( IF 6.9 ) Pub Date : 2023-05-03 , DOI: 10.1016/j.biopha.2023.114819
Yasmin S Abulfadl 1 , Yousef Abo El Ela 2 , Abdallah M Al Khaiyat 2 , Khalil I Elkhodary 2 , Mohamed Badran 2
Biomedicine & Pharmacotherapy ( IF 6.9 ) Pub Date : 2023-05-03 , DOI: 10.1016/j.biopha.2023.114819
Yasmin S Abulfadl 1 , Yousef Abo El Ela 2 , Abdallah M Al Khaiyat 2 , Khalil I Elkhodary 2 , Mohamed Badran 2
Affiliation
|
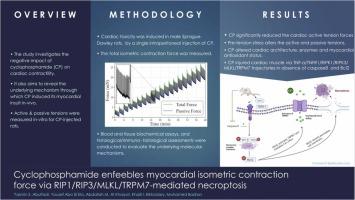
This study explores the negative impact of cyclophosphamide (CP) on cardiac contractility by specifically examining its effect on the active and passive tension of the cardiac muscle in-vitro and revealing the mechanism through which CP induces myocardial insult in-vivo. In young male Sprague-Dawley rats, cardiac toxicity was induced by a single intraperitoneal injection of CP (150 mg/kg body weight). Axial heart tissue slices were electrically stimulated, and the total isometric contraction force was measured at varying pretension levels. Blood and tissue biochemical assays, and histological/ immuno-histological assessments were conducted to evaluate the underlying molecular mechanisms. Statistical analysis shows that there is a significant difference between the drugged and the control groups in terms of the active tension values. Moreover, the pre-tension stress significantly affects both the active and passive tension values. CP altered heart, body, and heart-to-body weight, desolated cardiac muscle architecture, surged cardiac enzymes (CK-MB, LDH, and cTn l), augmented myocardial oxidative stressors (MDA), and weakened myocardial antioxidant status (SOD and GSH). Mechanistically, cyclophosphamide prompted the necroptotic trajectory evidenced by the activation of RIPK1, RIPK3, MLKL and TRPM7, the inhibition of caspase 8 and BCL2 and the upregulation of the protein/mRNA expression of TNF-α and TNFR1. This study identifies necroptosis as a key factor in cyclophosphamide-evoked myocardial contractility impairment, highlighting its potential as a target for alleviating antitumor-related myocardial damage. This innovative approach to investigating the underlying mechanisms of CP-induced cardiac toxicity offers valuable insights into the potential of developing new therapies to mitigate cyclophosphamide’s negative impact.
中文翻译:

环磷酰胺通过 RIP1/RIP3/MLKL/TRPM7 介导的坏死性凋亡削弱心肌等长收缩力
本研究通过具体考察环磷酰胺(CP)在体外对心肌主动和被动张力的影响,探讨环磷酰胺(CP)对心肌收缩力的负面影响,并揭示环磷酰胺在体内诱发心肌损伤的机制。在年轻雄性 Sprague-Dawley 大鼠中,通过单次腹腔注射 CP(150 mg/kg 体重)诱导心脏毒性。对轴向心脏组织切片进行电刺激,并在不同的预张力水平下测量总等长收缩力。进行血液和组织生化测定以及组织学/免疫组织学评估以评估潜在的分子机制。统计分析表明,给药组与对照组的主动张力值存在显着差异。此外,预应力显着影响主动和被动张力值。 CP改变了心脏、身体和心脏与身体之间的重量,心肌结构退化,心肌酶(CK-MB、LDH和cTn l)激增,心肌氧化应激源(MDA)增加,心肌抗氧化状态(SOD和SOD)减弱。还原型谷胱甘肽)。从机制上讲,环磷酰胺促进了坏死性凋亡轨迹,其证据是激活 RIPK1、RIPK3、MLKL 和 TRPM7、抑制 caspase 8 和 BCL2 以及上调 TNF-α 和 TNFR1 的蛋白/mRNA 表达。这项研究将坏死性凋亡确定为环磷酰胺引起的心肌收缩力损伤的关键因素,强调了其作为减轻抗肿瘤相关心肌损伤的目标的潜力。 这种研究 CP 引起的心脏毒性的潜在机制的创新方法为开发新疗法以减轻环磷酰胺的负面影响的潜力提供了宝贵的见解。
更新日期:2023-05-03
中文翻译:

环磷酰胺通过 RIP1/RIP3/MLKL/TRPM7 介导的坏死性凋亡削弱心肌等长收缩力
本研究通过具体考察环磷酰胺(CP)在体外对心肌主动和被动张力的影响,探讨环磷酰胺(CP)对心肌收缩力的负面影响,并揭示环磷酰胺在体内诱发心肌损伤的机制。在年轻雄性 Sprague-Dawley 大鼠中,通过单次腹腔注射 CP(150 mg/kg 体重)诱导心脏毒性。对轴向心脏组织切片进行电刺激,并在不同的预张力水平下测量总等长收缩力。进行血液和组织生化测定以及组织学/免疫组织学评估以评估潜在的分子机制。统计分析表明,给药组与对照组的主动张力值存在显着差异。此外,预应力显着影响主动和被动张力值。 CP改变了心脏、身体和心脏与身体之间的重量,心肌结构退化,心肌酶(CK-MB、LDH和cTn l)激增,心肌氧化应激源(MDA)增加,心肌抗氧化状态(SOD和SOD)减弱。还原型谷胱甘肽)。从机制上讲,环磷酰胺促进了坏死性凋亡轨迹,其证据是激活 RIPK1、RIPK3、MLKL 和 TRPM7、抑制 caspase 8 和 BCL2 以及上调 TNF-α 和 TNFR1 的蛋白/mRNA 表达。这项研究将坏死性凋亡确定为环磷酰胺引起的心肌收缩力损伤的关键因素,强调了其作为减轻抗肿瘤相关心肌损伤的目标的潜力。 这种研究 CP 引起的心脏毒性的潜在机制的创新方法为开发新疗法以减轻环磷酰胺的负面影响的潜力提供了宝贵的见解。

































 京公网安备 11010802027423号
京公网安备 11010802027423号