当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of a Potent and Oral Available Complex I OXPHOS Inhibitor That Abrogates Tumor Growth and Circumvents MEKi Resistance
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2023-05-02 , DOI: 10.1021/acs.jmedchem.2c01844 Peng He 1 , Juanjuan Feng 1, 2 , Xinting Xia 1 , Yue Sun 1 , Jia He 1 , Tian Guan 1 , Yangrui Peng 1 , Xueli Zhang 2 , Mingyao Liu 1 , Xiufeng Pang 1 , Yihua Chen 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2023-05-02 , DOI: 10.1021/acs.jmedchem.2c01844 Peng He 1 , Juanjuan Feng 1, 2 , Xinting Xia 1 , Yue Sun 1 , Jia He 1 , Tian Guan 1 , Yangrui Peng 1 , Xueli Zhang 2 , Mingyao Liu 1 , Xiufeng Pang 1 , Yihua Chen 1
Affiliation
|
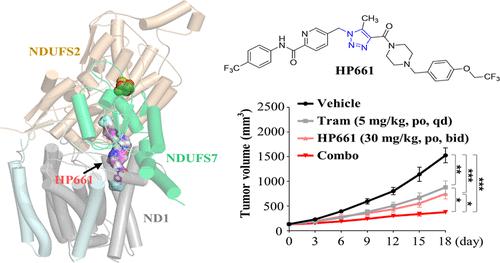
Targeting oxidative phosphorylation (OXPHOS) has emerged as a promising therapeutic strategy for cancer therapy. Here, we discovered a 1H-1,2,3-triazole derivative HP661 as a highly potent and orally available OXPHOS inhibitor that effectively blocked the activity of mitochondrial complex I. HP661 specifically compromised the mitochondrial oxygen consumption of high-OXPHOS lung cancer cells but not that of low-OXPHOS lung cancer cells or normal cells in the low nanomolar range. Notably, mitogen-activated protein kinase kinase (MEK) inhibitor (trametinib)-resistant lung cancer cells with high levels of OXPHOS also showed marked sensitivity to HP661, as indicated by decreased clonogenic growth and increased cell apoptosis upon treatment. In a mouse model of high-OXPHOS lung cancer, HP661 treatment not only significantly suppressed tumor growth but also augmented the therapeutic efficacy of trametinib by impairing tumor mitochondrial respiration. In summary, we identified HP661 as a highly effective OXPHOS inhibitor to abrogate the growth of high OXPHOS-dependent tumors and conquer high OXPHOS-mediated drug resistance.
中文翻译:

发现一种有效的口服复合物 I OXPHOS 抑制剂,可消除肿瘤生长并规避 MEKi 耐药性
靶向氧化磷酸化(OXPHOS)已成为一种有前途的癌症治疗策略。在这里,我们发现了一种 1 H -1,2,3-三唑衍生物HP661作为一种高效、口服的 OXPHOS 抑制剂,可有效阻断线粒体复合物 I 的活性。HP661特别损害高 OXPHOS 肺癌细胞的线粒体耗氧量但低 OXPHOS 肺癌细胞或低纳摩尔范围内的正常细胞则不然。值得注意的是,具有高水平 OXPHOS 的丝裂原激活蛋白激酶激酶 (MEK) 抑制剂 (trametinib) 耐药的肺癌细胞也表现出对HP661 的显着敏感性,如治疗后克隆生长减少和细胞凋亡增加所示。在高 OXPHOS 肺癌小鼠模型中,HP661治疗不仅显着抑制肿瘤生长,还通过损害肿瘤线粒体呼吸来增强曲美替尼的治疗效果。总之,我们确定HP661是一种高效的 OXPHOS 抑制剂,可以消除高 OXPHOS 依赖性肿瘤的生长并克服高 OXPHOS 介导的耐药性。
更新日期:2023-05-02
中文翻译:

发现一种有效的口服复合物 I OXPHOS 抑制剂,可消除肿瘤生长并规避 MEKi 耐药性
靶向氧化磷酸化(OXPHOS)已成为一种有前途的癌症治疗策略。在这里,我们发现了一种 1 H -1,2,3-三唑衍生物HP661作为一种高效、口服的 OXPHOS 抑制剂,可有效阻断线粒体复合物 I 的活性。HP661特别损害高 OXPHOS 肺癌细胞的线粒体耗氧量但低 OXPHOS 肺癌细胞或低纳摩尔范围内的正常细胞则不然。值得注意的是,具有高水平 OXPHOS 的丝裂原激活蛋白激酶激酶 (MEK) 抑制剂 (trametinib) 耐药的肺癌细胞也表现出对HP661 的显着敏感性,如治疗后克隆生长减少和细胞凋亡增加所示。在高 OXPHOS 肺癌小鼠模型中,HP661治疗不仅显着抑制肿瘤生长,还通过损害肿瘤线粒体呼吸来增强曲美替尼的治疗效果。总之,我们确定HP661是一种高效的 OXPHOS 抑制剂,可以消除高 OXPHOS 依赖性肿瘤的生长并克服高 OXPHOS 介导的耐药性。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号