当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced Nitrate Reduction Activity from Cu-Alloy Electrodes in an Alkaline Electrolyte
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-05-02 , DOI: 10.1021/acscatal.3c00999 Akhil Paliwal 1 , Christopher D. Bandas 1 , Eric S. Thornburg 1 , Richard T. Haasch 2 , Andrew A. Gewirth 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-05-02 , DOI: 10.1021/acscatal.3c00999 Akhil Paliwal 1 , Christopher D. Bandas 1 , Eric S. Thornburg 1 , Richard T. Haasch 2 , Andrew A. Gewirth 1, 2
Affiliation
|
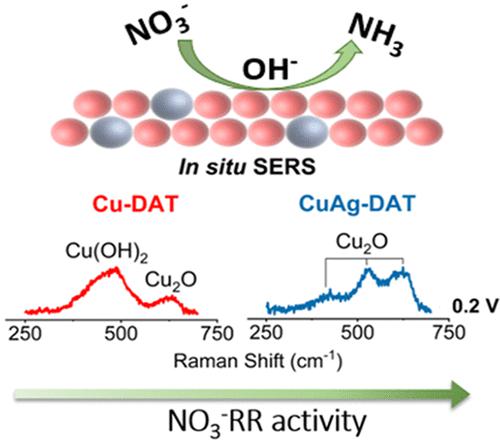
The electrochemical nitrate reduction reaction (NO3–RR) offers two-fold advantages─restoring balance to the global nitrogen cycle and a less energy intensive pathway to the production of ammonia. We report the results of voltammetric and spectroscopic measurements examining NO3–RR on Cu and Cu-alloyed electrodes (CuAg, CuSn, and CuPt) in an alkaline medium. Electrochemical results demonstrate that the overpotential for the NO3–RR is ∼120 mV less on the CuAg catalyst as compared to the Cu-only catalyst. In situ surface enhanced Raman spectroscopy (SERS) obtained from these two Cu samples shows that the presence of dilute Ag maintains the Cu surface in a more reduced state (Cu(I)) during the course of NO3–RR, while the neat Cu surface is heavily oxidized during NO3–RR in an alkaline medium. Consistent with this behavior, the CuSn alloy also stabilizes Cu(I) on the electrode surface and results in increased NO3–RR rates. Alternatively, the CuPt alloy does not yield a stabilized Cu(I) component and consequently results in NO3–RR rates lower than those for neat Cu. These results indicate that alloying Cu with different metals can tune the nitrate reduction activity by making the Cu atoms more resistant to oxidation to Cu(II) and stabilizing the Cu atoms in lower oxidation states.
中文翻译:

碱性电解质中铜合金电极增强的硝酸盐还原活性
电化学硝酸盐还原反应 (NO 3 – RR) 具有双重优势 ─ 恢复全球氮循环的平衡和减少氨生产的能源密集型途径。我们报告了在碱性介质中对铜和铜合金电极(CuAg、CuSn 和 CuPt)检测 NO 3 – RR的伏安法和光谱测量结果。电化学结果表明,与仅含 Cu 的催化剂相比,CuAg 催化剂上NO 3 – RR 的过电势要小 ~ 120 mV。从这两个 Cu 样品获得的原位表面增强拉曼光谱 (SERS) 表明,在 NO 3– RR,而纯 Cu 表面在 NO 3 – RR 期间在碱性介质中被严重氧化。与此行为一致,CuSn 合金还稳定了电极表面上的 Cu(I) 并导致增加的 NO 3 – RR 率。或者,CuPt 合金不会产生稳定的 Cu(I) 成分,因此导致 NO 3 – RR 率低于纯 Cu。这些结果表明,将 Cu 与不同金属合金化可以通过使 Cu 原子更耐氧化为 Cu(II) 并将 Cu 原子稳定在较低的氧化态来调节硝酸盐还原活性。
更新日期:2023-05-02
中文翻译:

碱性电解质中铜合金电极增强的硝酸盐还原活性
电化学硝酸盐还原反应 (NO 3 – RR) 具有双重优势 ─ 恢复全球氮循环的平衡和减少氨生产的能源密集型途径。我们报告了在碱性介质中对铜和铜合金电极(CuAg、CuSn 和 CuPt)检测 NO 3 – RR的伏安法和光谱测量结果。电化学结果表明,与仅含 Cu 的催化剂相比,CuAg 催化剂上NO 3 – RR 的过电势要小 ~ 120 mV。从这两个 Cu 样品获得的原位表面增强拉曼光谱 (SERS) 表明,在 NO 3– RR,而纯 Cu 表面在 NO 3 – RR 期间在碱性介质中被严重氧化。与此行为一致,CuSn 合金还稳定了电极表面上的 Cu(I) 并导致增加的 NO 3 – RR 率。或者,CuPt 合金不会产生稳定的 Cu(I) 成分,因此导致 NO 3 – RR 率低于纯 Cu。这些结果表明,将 Cu 与不同金属合金化可以通过使 Cu 原子更耐氧化为 Cu(II) 并将 Cu 原子稳定在较低的氧化态来调节硝酸盐还原活性。































 京公网安备 11010802027423号
京公网安备 11010802027423号