Redox Biology ( IF 10.7 ) Pub Date : 2023-05-02 , DOI: 10.1016/j.redox.2023.102727 Limin Shi 1 , Zhipeng Tao 2 , Louise Zheng 3 , Jinying Yang 4 , Xinran Hu 5 , Karen Scott 6 , Annette de Kloet 7 , Eric Krause 6 , James F Collins 4 , Zhiyong Cheng 8
|
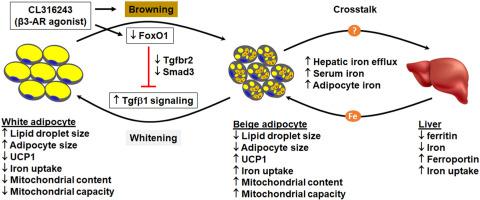
脂肪可塑性对代谢稳态至关重要。脂肪细胞转分化在脂肪可塑性中起重要作用,但转分化的分子机制仍不完全清楚。在这里,我们表明转录因子 FoxO1 通过介导 Tgfβ1 信号通路调节脂肪转分化。Tgfβ1 处理诱导米色脂肪细胞变白表型,降低 UCP1 和线粒体容量并扩大脂滴。脂肪 FoxO1 (adO1KO) 的缺失通过下调 Tgfbr2 和 Smad3 抑制 Tgfβ1 信号,并诱导小鼠脂肪组织褐变,增加 UCP1 和线粒体含量并激活代谢途径。沉默 FoxO1 也消除了 Tgfβ1 对米色脂肪细胞的美白作用。与对照小鼠相比,adO1KO 小鼠表现出明显更高的能量消耗、更低的脂肪量和更小的脂肪细胞。adO1KO 小鼠的褐变表型与脂肪组织中铁含量增加有关,同时上调促进铁摄取的蛋白质(DMT1 和 TfR1)和铁输入线粒体(Mfrn1)。对 adO1KO 小鼠的肝脏和血清铁以及肝脏铁调节蛋白(铁蛋白和铁转运蛋白)的分析表明,脂肪组织-肝脏串扰满足脂肪褐变增加的铁需求。FoxO1-Tgfβ1 信号级联也是由 β3-AR 激动剂 CL316243 诱导的脂肪褐变的基础。我们的研究提供了 FoxO1-Tgfβ1 轴在调节脂肪褐变-增白转分化和铁内流中的第一个证据,这揭示了在 FoxO1 和 Tgfβ1 信号失调的情况下脂肪可塑性受损。对 adO1KO 小鼠的肝脏和血清铁以及肝脏铁调节蛋白(铁蛋白和铁转运蛋白)的分析表明,脂肪组织-肝脏串扰满足脂肪褐变增加的铁需求。FoxO1-Tgfβ1 信号级联也是由 β3-AR 激动剂 CL316243 诱导的脂肪褐变的基础。我们的研究提供了 FoxO1-Tgfβ1 轴在调节脂肪褐变-增白转分化和铁内流中的第一个证据,这揭示了在 FoxO1 和 Tgfβ1 信号失调的情况下脂肪可塑性受损。对 adO1KO 小鼠的肝脏和血清铁以及肝脏铁调节蛋白(铁蛋白和铁转运蛋白)的分析表明,脂肪组织-肝脏串扰满足脂肪褐变增加的铁需求。FoxO1-Tgfβ1 信号级联也是由 β3-AR 激动剂 CL316243 诱导的脂肪褐变的基础。我们的研究提供了 FoxO1-Tgfβ1 轴在调节脂肪褐变-增白转分化和铁内流中的第一个证据,这揭示了在 FoxO1 和 Tgfβ1 信号失调的情况下脂肪可塑性受损。

"点击查看英文标题和摘要"






























 京公网安备 11010802027423号
京公网安备 11010802027423号