Chemical Physics Letters ( IF 2.8 ) Pub Date : 2023-05-02 , DOI: 10.1016/j.cplett.2023.140565 Shiduo Zhang , Wenlong Yang , Minyi Zhang
|
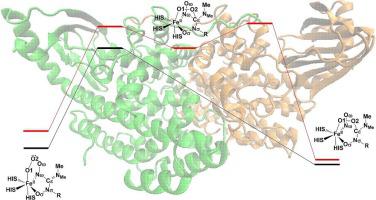
The SznF enzyme provides a novel approach to synthesize the N-nitrosourea pharmacophore. In this work, the modified L-Arg with methyl nitrogen deprotonation(L-DHMAd), a not yet studied substrate’s reaction mechanism of the critical N-nitrosation reaction catalyzed by the cupin domain of SznF enzyme is investigated, through the combined quantum mechanics/molecular mechanics method. Basis on our calculation results, the optimal N-nitrosation reaction pathway can be divided in to three reaction steps:1) superoxo addition with accompanying of Cε-Nω bond cleavage; 2) O1-O2 bond heterolytic cleavage; 3) NMe-Nω bond coupling. The first reaction step is the rate-determining step of the entire optimal reaction with an energy barrier of 20.6 Kcal/mol. Significantly, Cε-Nω bond could be homolytic cleaved spontaneously during the Cε-O2 bond formation in the first reaction step. This appearance mainly attributes to the Cε-Nω single bond of the L-DHMAd, which could be more feasible to cleave to maintain the Cε sp2 hybridization within the superoxo addition. The subsequent reaction step is the O1-O2 bond heterolytic cleavage, which involves a minimum energy crossing point to form the stable intermediate with Fe(IV) = O1 species at quintet state. Finally, NMe and Nω of L-DHMAd are very energetic favorable to coupling to form the N-nitroso product. This is consist with the experimental observations that two nitrogen atoms of the N-nitroso product are both from the same arginine substrate. Our work could contribute to the deeper understanding of the N-nitrosation reaction catalyzed by SznF enzyme, and might enlighten further studies of biomimetic chemistry of SznF enzyme.
中文翻译:

SznF酶cupin结构域催化甲基氮去质子修饰L-Arg N-亚硝化反应亚硝化反应机理的理论研究
SznF 酶提供了一种合成 N-亚硝基脲药效团的新方法。在这项工作中,通过组合量子力学研究了甲基氮去质子化修饰的L-Arg(L-DHMA d),一种尚未研究的底物在SznF酶的cupin结构域催化的临界N-亚硝化反应中的反应机制/分子力学方法。根据我们的计算结果,最佳的N-亚硝化反应途径可分为三个反应步骤:1)超氧加成伴随Cε-Nω键断裂;2) O1-O2键异裂解;3) N Me -N ω键耦合。第一反应步是整个最优反应的决速步,能垒为20.6 Kcal/mol。值得注意的是,Cε -N ω键可在第一步反应中 Cε -O2 键形成过程中自发均裂。这种现象主要归因于 L-DHMA d的 Cε-Nω 单键,它更容易切割以在超氧加成中维持 Cεsp2 杂交。随后的反应步骤是 O1-O2 键异裂裂解,它涉及一个最小能量交叉点,以在五重态下与 Fe(IV) = O1 物种形成稳定的中间体。最后,L-DHMA d的 N Me和 N ω非常有利于偶联形成N-亚硝基产物。这与实验观察结果一致,即 N-亚硝基产物的两个氮原子均来自同一精氨酸底物。我们的工作有助于加深对 SznF 酶催化的 N-亚硝化反应的理解,并可能启发对 SznF 酶的仿生化学的进一步研究。






























 京公网安备 11010802027423号
京公网安备 11010802027423号