当前位置:
X-MOL 学术
›
Chem. Eng. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NiO and Co3O4 nanoparticles decorated La0.8Sr0.2MnO3-based electrodes for electrochemical NOx removal in solid electrolyte cells
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2023-05-02 , DOI: 10.1016/j.cej.2023.143248
Jiabin Wang , Lei Ma , Wanting Tan , Shuai Wang , Junhui Wen , Zhezong Zhang , Honbing Yu , Wenjie Li
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2023-05-02 , DOI: 10.1016/j.cej.2023.143248
Jiabin Wang , Lei Ma , Wanting Tan , Shuai Wang , Junhui Wen , Zhezong Zhang , Honbing Yu , Wenjie Li
|
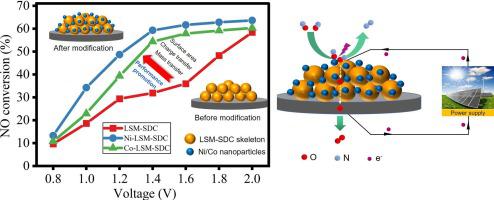
Solid electrolyte cells (SECs) have great application prospect for nitrogen oxides (NO) removal due to no required reductant addition in contrast to the conventional selective catalytic reduction (SCR) method. Perovskites are the potential electrode materials in SECs but their reaction activity is insufficient at the intermediate-low temperature. Herein, NiO and CoO nanoparticles are decorated on the LaSrMnO-based electrode to improve the electrode activity. A variety of characterizations were conducted to analyze the electrode properties, and the electrode performance was systematically evaluated. The results show that the decorated NiO and CoO nanoparticles both improved the electrode performance significantly and the NiO-modified electrode had a better performance. Compared with the unmodified electrodes, the surface NiO nanoparticles decreased the area-specific polarization resistance by 63.1% to 81.7 Ω cm while the NO conversion was increased by 85.9% to 59.3% at 1.4 V in 1000 ppm NO, 700 ℃. The electrochemical impedance spectra revealed that the nanoparticles not only promoted the species adsorption, dissociation and diffusion processes but facilitated the charge transfer between electrode and electrolyte. The first-principle calculation confirmed that the decorated NiO nanoparticles decreased the energy gap of electrodes and promoted the electron transfer between electrode and NO molecule, in agreement well with the experimental results. This work presented a new way to increase the NO removal performance of SECs and offered guidance for the future construction of high-performance electrodes.
中文翻译:

NiO 和 Co3O4 纳米颗粒装饰 La0.8Sr0.2MnO3 基电极,用于固体电解质电池中电化学 NOx 去除
与传统的选择性催化还原(SCR)方法相比,固体电解质电池(SEC)由于不需要添加还原剂,在氮氧化物(NO)去除方面具有巨大的应用前景。钙钛矿是SEC中潜在的电极材料,但其在中低温下的反应活性不足。在此,NiO和CoO纳米粒子被装饰在LaSrMnO基电极上以提高电极活性。进行了各种表征来分析电极性能,并对电极性能进行了系统评估。结果表明,修饰的NiO和CoO纳米粒子均显着改善了电极性能,其中NiO修饰的电极性能更好。与未修饰的电极相比,表面NiO纳米颗粒将面积极化电阻降低了63.1%至81.7 Ω·cm,而在1.4 V、1000 ppm NO、700 ℃下NO转化率提高了85.9%至59.3%。电化学阻抗谱表明,纳米粒子不仅促进了物质的吸附、解离和扩散过程,而且促进了电极和电解质之间的电荷转移。第一性原理计算证实,修饰的NiO纳米粒子减小了电极的能隙,促进了电极与NO分子之间的电子转移,与实验结果吻合良好。这项工作提出了一种提高SEC去除NO性能的新方法,并为未来高性能电极的构建提供了指导。
更新日期:2023-05-02
中文翻译:

NiO 和 Co3O4 纳米颗粒装饰 La0.8Sr0.2MnO3 基电极,用于固体电解质电池中电化学 NOx 去除
与传统的选择性催化还原(SCR)方法相比,固体电解质电池(SEC)由于不需要添加还原剂,在氮氧化物(NO)去除方面具有巨大的应用前景。钙钛矿是SEC中潜在的电极材料,但其在中低温下的反应活性不足。在此,NiO和CoO纳米粒子被装饰在LaSrMnO基电极上以提高电极活性。进行了各种表征来分析电极性能,并对电极性能进行了系统评估。结果表明,修饰的NiO和CoO纳米粒子均显着改善了电极性能,其中NiO修饰的电极性能更好。与未修饰的电极相比,表面NiO纳米颗粒将面积极化电阻降低了63.1%至81.7 Ω·cm,而在1.4 V、1000 ppm NO、700 ℃下NO转化率提高了85.9%至59.3%。电化学阻抗谱表明,纳米粒子不仅促进了物质的吸附、解离和扩散过程,而且促进了电极和电解质之间的电荷转移。第一性原理计算证实,修饰的NiO纳米粒子减小了电极的能隙,促进了电极与NO分子之间的电子转移,与实验结果吻合良好。这项工作提出了一种提高SEC去除NO性能的新方法,并为未来高性能电极的构建提供了指导。

































 京公网安备 11010802027423号
京公网安备 11010802027423号