Hellenic Journal of Cardiology ( IF 2.7 ) Pub Date : 2023-04-29 , DOI: 10.1016/j.hjc.2023.04.011 Basil Vasileios D Thanopoulos 1 , Georgios C Bompotis 2 , Dan Deleanou 3 , Petros Dardas 4 , Vlasis Ninios 5 , George S Tsaousis 1 , Athanasios Trikas 6 , Vasileios Saxpekidis 2
|
Background
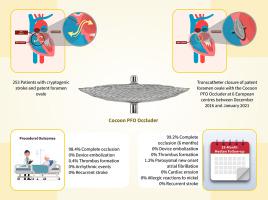
The Cocoon patent foramen ovale (PFO) occluder is a new device especially designed for transcatheter closure of PFO. This occluder has some distinctive structural modifications aimed at reducing the risk of major complications of transcatheter PFO closure. In this report we present our initial experience to evaluate the efficacy and safety of Cocoon PFO occluder in 253 patients who underwent transcatheter PFO closure.
Methods
The study cohort included 253 patients (median age 45 years) with embolic stroke of undetermined source who underwent attempted transcatheter closure of PFO for secondary prevention of paradoxical embolism. Patients were enrolled retrospectively from 5 sites in Greece and 1 in Romania between December-2016 and January-2021 and median follow–up period was 28 months (range 12–48 months). Clinical and laboratory data from each participating center were sent to an electronic registry for evaluation and statistical analysis.
Results
The Cocoon PFO occluder was permanently implanted in all (100%) patients. At 6 months, complete occlusion of PFO was observed in 251/253 (99.2%) patients. Three (1.2%) patients had a trivial residual shunt. Thrombus formation on the device which was successfully treated with recombinant tissue plasminogen activator infusion was observed in one (0.4%) patient. No other complications occurred. During a median follow-up period of 28 months, 3 (1.2 %) patients aged 64 – 67 years, developed paroxysmal new onset atrial fibrillation. No neurologic events, cardiac erosions, allergic reactions to nickel or thrombus formation occurred.
Conclusion
The Cocoon PFO occluder is an effective and safe device that adds to our armamentarium for transcatheter closure of PFO.
中文翻译:

使用 Cocoon 封堵器经导管封闭卵圆孔未闭:一项多中心回顾性研究
背景
Cocoon 卵圆孔未闭 (PFO) 封堵器是专为经导管封堵 PFO 而设计的新型装置。该封堵器具有一些独特的结构修改,旨在降低经导管 PFO 封堵的主要并发症的风险。在本报告中,我们介绍了我们在 253 名接受经导管 PFO 封堵术的患者中评估 Cocoon PFO 封堵器的有效性和安全性的初步经验。
方法
该研究队列包括 253 名不明原因栓塞性卒中患者(中位年龄 45 岁),他们尝试经导管封堵 PFO,以实现反常栓塞的二级预防。 2016 年 12 月至 2021 年 1 月期间,对希腊 5 个地点和罗马尼亚 1 个地点的患者进行回顾性入组,中位随访期为 28 个月(范围 12-48 个月)。每个参与中心的临床和实验室数据被发送到电子登记处进行评估和统计分析。
结果
Cocoon PFO 封堵器被永久植入所有(100%)患者体内。 6 个月时,251/253 (99.2%) 例患者观察到 PFO 完全闭塞。三名 (1.2%) 患者有轻微的残余分流。在一名 (0.4%) 患者中观察到使用重组组织纤溶酶原激活剂输注成功治疗的装置上出现血栓形成。没有其他并发症发生。在中位随访 28 个月期间,3 名 (1.2%) 年龄为 64-67 岁的患者出现阵发性新发房颤。没有发生神经系统事件、心脏糜烂、镍过敏反应或血栓形成。
结论
Cocoon PFO 封堵器是一种有效且安全的装置,为我们经导管封堵 PFO 提供了丰富的装备。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号