Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2023-04-29 , DOI: 10.1016/j.molstruc.2023.135685
D.L. Roopa , K. Shyamsunder , Prashantha Karunakar , Jothi Ramalingam Rajabathar , Adavala Venkatesulu , Muthusamy Karnan , K.S. Kiran , Manickam Selvaraj , S.M. Basavarajaiah
|
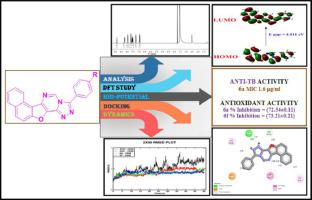
Napthofuran and its fused heterocyclic derivatives evaluated with varied biological activity functional groups comprise an important class of compounds for new chemical entities. We here in reporting synthesis of new 3-(4-substituted phenyl)naphtho[1′,2′:4,5]furo[2,3-e][1,2,4]triazolo[4,3-c]pyrimidines 6(a-f). Structures of the newly synthesized compounds were confirmed by making use of spectroscopic techniques like IR, NMR and Mass. The DFT calculations were taken for the selected molecules using B3LYP hybrid functional with a 6–31+G (d, p) all-electron basis set using the Gaussian 09 package. The bioactivity predictions were evaluated for the synthesized compounds. The In vitro biological activities were reported for the all compounds 6(a-f). The compound 6a showed high activity of anti-TB and antioxidant activity with at MIC 1.6 μg/ml and at percentage of inhibition (72.54±0.21) at 10μg/ml respectively. The compound 6f (73.21±0.11) showed antioxidant activity better than standard drug BHA (71.32±0.13) at 10 μg/ml. Furthermore, the docking studies for the newly synthesized molecules were carried out by Auto dock software with proteins InhA (4TZK),Cytochrome c peroxidase (2 × 08) and protease (Mpro) of SARS-CoV-2 Omicron (PDB ID: 7TOB). All the compounds showed a strong binding affinity for the docked proteins. The outcome of docking results showed that compound 6ahad excellent binding energies -10.8, -9.4, and -9.0 kcal/mol with 4TZK, 2 × 08, and 7TOB respectively. Lastly, the protein stability, fluctuations of APO-Protein, protein-ligand complexes were investigated through Molecular Dynamics (MD) simulations studies using Desmond Maestro 11.3 and potential lead molecules were identified.
中文翻译:

萘并[2,1-b]呋喃衍生的三唑嘧啶作为高潜力的 InhA 和细胞色素 c 过氧化物酶抑制剂:合成、DFT 计算、类药性、分子对接和动态研究
具有不同生物活性官能团的萘并呋喃及其稠合杂环衍生物构成了一类重要的新化学实体化合物。我们在这里报道了新的 3-(4-取代苯基)萘并 [1',2':4,5]furo[2,3- e ][1,2,4]triazolo[4,3- c ]的合成嘧啶6(af) . 通过使用红外、核磁共振和质谱等光谱技术确认新合成化合物的结构。使用具有 6–31+ G (d, p) 全电子基的 B3LYP 杂化泛函对所选分子进行DFT计算使用 Gaussian 09 包设置。对合成化合物的生物活性预测进行了评估。体外_报告了所有化合物6(af)的生物活性。化合物6a在MIC为1.6 μg/ml和10μg/ml时的抑制率分别为(72.54±0.21)时表现出较高的抗结核活性和抗氧化活性。化合物6f (73.21±0.11) 在 10 μg/ml 时表现出比标准药物 BHA (71.32±0.13) 更好的抗氧化活性。此外,新合成分子的对接研究是通过 Auto dock 软件与SARS-CoV-2 Omicron的蛋白质InhA (4TZK)、细胞色素 c 过氧化物酶(2×08) 和蛋白酶 (Mpro)进行的。(PDB ID:7TOB)。所有化合物都显示出对对接蛋白质的强结合亲和力。对接结果表明,化合物6a与4TZK、2×08和7TOB分别具有-10.8、-9.4和-9.0 kcal/mol的结合能。最后,使用 Desmond Maestro 11.3 通过分子动力学 (MD) 模拟研究研究了蛋白质稳定性、APO 蛋白质的波动、蛋白质-配体复合物,并确定了潜在的先导分子。

































 京公网安备 11010802027423号
京公网安备 11010802027423号