Journal of the Taiwan Institute of Chemical Engineers ( IF 5.5 ) Pub Date : 2023-04-29 , DOI: 10.1016/j.jtice.2023.104895 Sakun Preedavijitkul , Chaowat Autthanit , Supachai Jadsadajerm , Chombongkot Srijaroen , Piyasan Praserthdam , Bunjerd Jongsomjit
|
Background
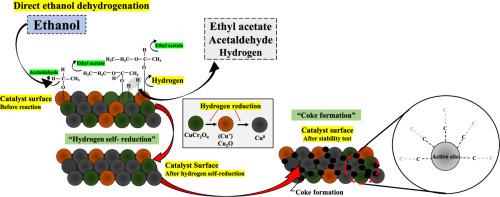
The utilization of bioethanol by converting it into additional high-value-added products including acetaldehyde, ethyl acetate, and hydrogen impresses as an appealing strategy at the present. The Cu-Cr catalysts improved product formation on ethanol dehydrogenation. This research was to assess the stability of the reaction and the deactivation of the catalyst over protracted ethanol dehydrogenation.
Methods
The deactivation behaviors of 50 Cu-Cr catalyst on ethanol dehydrogenation reaction was investigated. The co-precipitation technique was used to synthesize the Cu-Cr catalyst, which was then reduced by a mixture of H2/N2 prior to the stability test in the ethanol dehydrogenation reaction. The fresh, reduced, and/or spent catalysts after being used in ethanol dehydrogenation were characterized using XRD, SEM-EDX, HR-TEM, H2-TPR, NH3-TPD, XPS, TGA, and TPO.
Significant finding
The proportion of CuCr2O4, Cu+, and Cu0 active sites on the catalyst sample influenced catalytic activity. Based on the results, the activity was related to the oxidation state of copper-chromium in the Cu-Cr catalyst. Fascinatingly, the results of the ethanol dehydrogenation showed that the catalyst deactivation was caused by the change in the copper-chromium oxidation state via the hydrogen self-reduction process and the soft coke formation on active sites after prolonged ethanol dehydrogenation resulting in a decrease in catalytic activity.
中文翻译:

乙醇直接脱氢制乙酸乙酯、乙醛和氢气的Cu-Cr催化剂失活研究
背景
通过将生物乙醇转化为额外的高附加值产品(包括乙醛、乙酸乙酯和氢气)来利用生物乙醇是目前极具吸引力的策略。Cu-Cr 催化剂改善了乙醇脱氢的产物形成。本研究旨在评估反应的稳定性和催化剂在长期乙醇脱氢过程中的失活情况。
方法
研究了 50 Cu-Cr催化剂在乙醇脱氢反应中的失活行为。共沉淀技术用于合成Cu-Cr催化剂,然后在乙醇脱氢反应中的稳定性测试之前用H 2 /N 2的混合物还原。使用 XRD、SEM-EDX、HR-TEM、H 2 -TPR、NH 3 -TPD、XPS、TGA 和 TPO 对用于乙醇脱氢后的新鲜、还原和/或废催化剂进行表征。
重大发现
催化剂样品上CuCr 2 O 4、Cu +和Cu 0活性位点的比例影响催化活性。根据这些结果,活性与 Cu-Cr 催化剂中铜铬的氧化态有关。令人着迷的是,乙醇脱氢的结果表明,催化剂失活是由于氢气自还原过程中铜铬氧化态的变化以及乙醇脱氢时间延长后活性位点上软焦的形成导致催化性能降低引起的。活动。

































 京公网安备 11010802027423号
京公网安备 11010802027423号