Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self-Assembled Copper-Based Nanoparticles for Glutathione Activated and Enzymatic Cascade-Enhanced Ferroptosis and Immunotherapy in Cancer Treatment
Small ( IF 13.0 ) Pub Date : 2023-04-28 , DOI: 10.1002/smll.202301148 Wen-Fang Song 1 , Jin-Yue Zeng 1 , Ping Ji 1 , Zi-Yi Han 1 , Yun-Xia Sun 1 , Xian-Zheng Zhang 1, 2
Small ( IF 13.0 ) Pub Date : 2023-04-28 , DOI: 10.1002/smll.202301148 Wen-Fang Song 1 , Jin-Yue Zeng 1 , Ping Ji 1 , Zi-Yi Han 1 , Yun-Xia Sun 1 , Xian-Zheng Zhang 1, 2
Affiliation
|
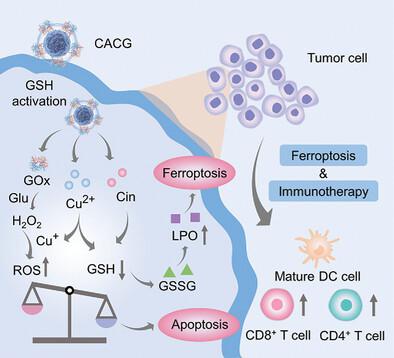
As an emerging cancer treatment strategy, ferroptosis is greatly restricted by excessive glutathione (GSH) in tumor microenvironment (TME) and low reactive oxygen species (ROS) generation efficiency. Here, this work designs self-assembled copper-alanine nanoparticles (CACG) loaded with glucose oxidase (GOx) and cinnamaldehyde (Cin) for in situ glutathione activated and enzymatic cascade-enhanced ferroptosis and immunotherapy. In response to GSH-rich and acidic TME, CACG allows to effectively co-deliver Cu2+, Cin, and GOx into tumors. Released Cin consumes GSH through Michael addition, accompanying with the reduction of Cu2+ into Cu+ for further GSH depletion. With the cascade of Cu+-catalyzed Fenton reactions and enzyme-catalyzed reactions by GOx, CACG could get rid of the restriction of insufficient hydrogen peroxide in TME, leading to a robust and constant generation of ROS. With the high efficiency of GSH depletion and ROS production, ferroptosis is significantly enhanced by CACG in vivo. Moreover, elevated oxidative stress triggers robust immune responses by promoting dendritic cells maturation and T cell infiltration. The in vivo results prove that CACG could efficiently inhibit tumor growth in 4T1 tumor-bearing mouse model without causing obvious systemic toxicity, suggesting the great potential of CACG in enhancing ferroptosis and immunotherapy for effective cancer treatment.
中文翻译:

用于癌症治疗中谷胱甘肽激活和酶级联增强铁死亡和免疫疗法的自组装铜基纳米颗粒
作为一种新兴的癌症治疗策略,铁死亡受到肿瘤微环境(TME)中过量的谷胱甘肽(GSH)和低活性氧(ROS)生成效率的极大限制。在这里,这项工作设计了负载葡萄糖氧化酶(GOx)和肉桂醛(Cin)的自组装铜丙氨酸纳米颗粒(CACG),用于原位谷胱甘肽激活和酶级联增强的铁死亡和免疫治疗。为了响应富含 GSH 和酸性的 TME,CACG 可以有效地将 Cu 2+、Cin 和 GOx共同递送到肿瘤中。释放的Cin通过Michael加成消耗GSH,同时将Cu 2+还原成Cu +进一步消耗GSH。通过Cu +催化的Fenton反应和GOx酶催化反应的级联,CACG可以摆脱TME中过氧化氢不足的限制,从而导致ROS的强劲且持续的产生。由于 GSH 消耗和 ROS 产生的高效率,CACG 在体内显着增强铁死亡。此外,氧化应激升高可促进树突状细胞成熟和 T 细胞浸润,从而引发强大的免疫反应。体内结果证明CACG可以有效抑制4T1荷瘤小鼠模型中的肿瘤生长,且不会引起明显的全身毒性,这表明CACG在增强铁死亡和有效癌症治疗的免疫治疗方面具有巨大潜力。
更新日期:2023-04-28
中文翻译:

用于癌症治疗中谷胱甘肽激活和酶级联增强铁死亡和免疫疗法的自组装铜基纳米颗粒
作为一种新兴的癌症治疗策略,铁死亡受到肿瘤微环境(TME)中过量的谷胱甘肽(GSH)和低活性氧(ROS)生成效率的极大限制。在这里,这项工作设计了负载葡萄糖氧化酶(GOx)和肉桂醛(Cin)的自组装铜丙氨酸纳米颗粒(CACG),用于原位谷胱甘肽激活和酶级联增强的铁死亡和免疫治疗。为了响应富含 GSH 和酸性的 TME,CACG 可以有效地将 Cu 2+、Cin 和 GOx共同递送到肿瘤中。释放的Cin通过Michael加成消耗GSH,同时将Cu 2+还原成Cu +进一步消耗GSH。通过Cu +催化的Fenton反应和GOx酶催化反应的级联,CACG可以摆脱TME中过氧化氢不足的限制,从而导致ROS的强劲且持续的产生。由于 GSH 消耗和 ROS 产生的高效率,CACG 在体内显着增强铁死亡。此外,氧化应激升高可促进树突状细胞成熟和 T 细胞浸润,从而引发强大的免疫反应。体内结果证明CACG可以有效抑制4T1荷瘤小鼠模型中的肿瘤生长,且不会引起明显的全身毒性,这表明CACG在增强铁死亡和有效癌症治疗的免疫治疗方面具有巨大潜力。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号