Nano Research ( IF 9.5 ) Pub Date : 2023-04-28 , DOI: 10.1007/s12274-023-5682-2 Zhikang Bao , Jinyan Zhao , Shijie Zhang , Xiaoge Peng , Yizhen Shao , Chenghang Jiang , Zaixiang Xu , Xing Zhong , Zihao Yao , Jianguo Wang
|
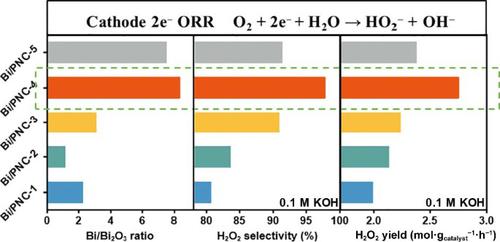
Electrocatalytic two-electron oxygen reduction reaction (2e− ORR) is a promising method for producing green and sustainable H2O2 but lacks high selectivity and yields electrocatalysts. And it is critical to develop catalysts that meet industrial demands. Herein, we report the different ratios of Bi0/Bi3+ supported on a phosphorus, nitrogen, and carbon nanosheet (Bi/PNC), which can reduce O2 to H2O2 with high selectivity (up to 97.75% at 0.4 VRHE) in 0.1 M KOH electrolyte and retain 97% selectivity even after 100 h electrolysis. Then a homemade flow-cell system was built for electrocatalytic production of H2O2 under an O2 atmosphere using an improved gas diffusion electrode. The Bi/PNC-4 can achieve a high H2O2 yield of 2.76 mol·g −1catalyst ·h−1 (alkaline), 5.29 mol·g −1catalyst ·h−1 (neutral), and 3.50 mol·g −1catalyst ·h−1 (acid) in universal pH conditions. The in-situ generated H2O2 can function as a degradation agent for efficiently degrading pesticides and antibiotics. The outstanding selectivity and activities are attributed to the synergistic effects of Bi0 and Bi3+ that promote proton-coupled reduction of O2 to OOH* (ΔGOOH* = 4.27 eV), and the formation of H2O2. The fast yield of H2O2 on Bi/PNC catalysts in flow-cell provides a promising path of electrocatalytic 2e− ORR for practical H2O2 production.
中文翻译:

调整 Bi/PNC 纳米片中 Bi/Bi2O3 的比例以实现高效电合成过氧化氢
电催化双电子氧还原反应 (2e − ORR) 是一种很有前途的生产绿色和可持续 H 2 O 2的方法,但缺乏高选择性和产量电催化剂。开发满足工业需求的催化剂至关重要。在此,我们报告了负载在磷、氮和碳纳米片 (Bi/PNC) 上的不同比例的 Bi 0 /Bi 3+ ,它可以将 O 2还原为 H 2 O 2并具有高选择性(在 0.4 时高达 97.75%) V RHE ) 在 0.1 M KOH 电解质中,即使在电解 100 小时后仍能保持 97% 的选择性。然后构建了一个自制的流通池系统,用于电催化生产 H2 O 2在O 2气氛下使用改进的气体扩散电极。Bi/PNC-4 可实现2.76 mol·g -1催化剂·h -1(碱性)、5.29 mol·g -1催化剂·h -1(中性)和 3.50 mol·的高 H 2 O 2产率g −1催化剂·h −1(酸)在通用 pH 条件下。原位生成的H 2 O 2 可作为高效降解农药和抗生素的降解剂。出色的选择性和活性归因于 Bi 0和 Bi 3+的协同作用,促进 O 2质子偶联还原为 OOH*(Δ G OOH* = 4.27 eV),以及 H 2 O 2的形成。流通池中 Bi/PNC 催化剂上H 2 O 2的快速产率为实际 H 2 O 2生产提供了电催化 2e - ORR 的有前景的途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号