当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrogen-Donor-Controlled Polybrominated Dibenzofuran (PBDF) Formation from Polybrominated Diphenyl Ether (PBDE) Photolysis in Solutions: Competition Mechanisms of Radical-Based Cyclization and Hydrogen Abstraction Reactions
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2023-04-28 , DOI: 10.1021/acs.est.2c08003 Xiaodong Du 1 , Haoliang Li 1 , Jiahao Liang 1 , Rui Wang 2 , Kaibo Huang 3 , Waseem Hayat 1 , Limiao Cai 1 , Xueqin Tao 4 , Zhi Dang 1, 5, 6 , Guining Lu 1, 5
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2023-04-28 , DOI: 10.1021/acs.est.2c08003 Xiaodong Du 1 , Haoliang Li 1 , Jiahao Liang 1 , Rui Wang 2 , Kaibo Huang 3 , Waseem Hayat 1 , Limiao Cai 1 , Xueqin Tao 4 , Zhi Dang 1, 5, 6 , Guining Lu 1, 5
Affiliation
|
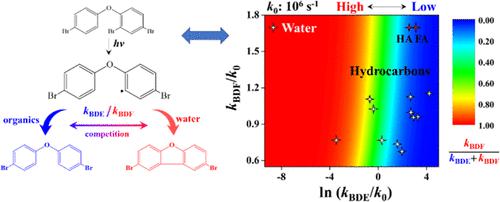
Polybrominated dibenzofurans (PBDFs) are characteristic dioxin-like products of polybrominated diphenyl ether (PBDE) photolysis. In this study, competition mechanisms of radical-based cyclization and hydrogen abstraction reactions are proposed in PBDF formation. Commonly, the ortho C–Br bond dissociation during photolysis generates aryl radicals, which undergo intramolecular cyclization to form PBDFs or hydrogen abstraction with hydrogen donors (such as organic solvents and water) to form lower brominated PBDEs. By using 2,4,4′-tribromodiphenyl ether (BDE-28) as the model reactant, the experimental PBDF formation ratios in various solutions are explained quantitatively by the calculated rate constants of cyclization and hydrogen abstraction reactions using the density functional theory (DFT) method. The solvent effect of pure and mixed solvents on PBDF formation is illustrated successfully. The structure-related hydrogen donation ability for hydrogen abstraction controls the bias of competition reactions and influences PBDF formation. Water resulted to be the most significant generation of PBDFs. Fulvic and humic acid display higher hydrogen donation ability than small-molecule organics due to the partitioning effect in aqueous solution. Quantitative structure–activity relationship (QSAR) models of the calculated rate constants for 512 cyclization and 319 hydrogen abstraction reactions using 189 PBDEs as the initial reactants in water are established, revealing the high risk of PBDF formation in aqueous solution.
中文翻译:

溶液中多溴二苯醚 (PBDE) 光解的氢供体控制多溴二苯并呋喃 (PBDF) 的形成:基于自由基的环化和夺氢反应的竞争机制
多溴二苯并呋喃 (PBDF) 是多溴二苯醚 (PBDE) 光解的典型二恶英类产物。在这项研究中,提出了 PBDF 形成中基于自由基的环化和夺氢反应的竞争机制。通常,正交C-Br键在光解过程中解离产生芳基自由基,芳基自由基在分子内环化形成PBDFs或与氢供体(如有机溶剂和水)夺氢形成低溴化PBDEs。通过使用 2,4,4'-三溴二苯醚 (BDE-28) 作为模型反应物,通过使用密度泛函理论 (DFT) 计算的环化和夺氢反应的速率常数来定量解释各种溶液中的实验 PBDF 形成率) 方法。成功地说明了纯溶剂和混合溶剂对 PBDF 形成的溶剂效应。结构相关的氢提取能力控制竞争反应的偏向并影响 PBDF 的形成。结果水是最重要的 PBDF 生成。由于水溶液中的分配效应,黄腐酸和腐植酸比小分子有机物具有更高的供氢能力。建立了使用 189 PBDEs 作为水中初始反应物的 512 环化和 319 氢提取反应的计算速率常数的定量构效关系 (QSAR) 模型,揭示了水溶液中 PBDF 形成的高风险。
更新日期:2023-04-28
中文翻译:

溶液中多溴二苯醚 (PBDE) 光解的氢供体控制多溴二苯并呋喃 (PBDF) 的形成:基于自由基的环化和夺氢反应的竞争机制
多溴二苯并呋喃 (PBDF) 是多溴二苯醚 (PBDE) 光解的典型二恶英类产物。在这项研究中,提出了 PBDF 形成中基于自由基的环化和夺氢反应的竞争机制。通常,正交C-Br键在光解过程中解离产生芳基自由基,芳基自由基在分子内环化形成PBDFs或与氢供体(如有机溶剂和水)夺氢形成低溴化PBDEs。通过使用 2,4,4'-三溴二苯醚 (BDE-28) 作为模型反应物,通过使用密度泛函理论 (DFT) 计算的环化和夺氢反应的速率常数来定量解释各种溶液中的实验 PBDF 形成率) 方法。成功地说明了纯溶剂和混合溶剂对 PBDF 形成的溶剂效应。结构相关的氢提取能力控制竞争反应的偏向并影响 PBDF 的形成。结果水是最重要的 PBDF 生成。由于水溶液中的分配效应,黄腐酸和腐植酸比小分子有机物具有更高的供氢能力。建立了使用 189 PBDEs 作为水中初始反应物的 512 环化和 319 氢提取反应的计算速率常数的定量构效关系 (QSAR) 模型,揭示了水溶液中 PBDF 形成的高风险。















































 京公网安备 11010802027423号
京公网安备 11010802027423号