当前位置:
X-MOL 学术
›
Drug Test. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Determination of amphetamine enantiomers in urine by conductive vial electromembrane extraction and ultra-high performance supercritical fluid chromatography tandem mass spectrometry
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2023-04-28 , DOI: 10.1002/dta.3487 Tonje Gottenberg Skaalvik 1, 2 , Elisabeth Leere Øiestad 2, 3 , Stig Pedersen-Bjergaard 2, 4 , Solfrid Hegstad 1
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2023-04-28 , DOI: 10.1002/dta.3487 Tonje Gottenberg Skaalvik 1, 2 , Elisabeth Leere Øiestad 2, 3 , Stig Pedersen-Bjergaard 2, 4 , Solfrid Hegstad 1
Affiliation
|
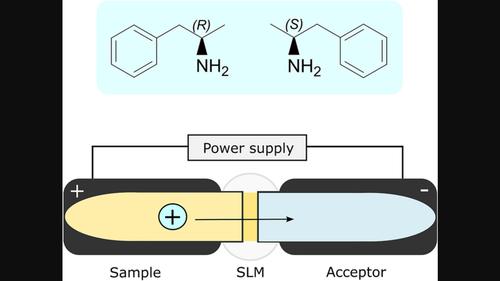
Separation and quantification of amphetamine enantiomers are commonly used to distinguish between consumption of prescription amphetamine (mostly S-amphetamine) and illicit forms of the drug (racemate). In this study, electromembrane extraction with prototype conductive vials was combined with ultra-high performance supercritical fluid chromatography (UHPSFC-MS/MS) to quantify R- and S-amphetamine in urine. Amphetamine was extracted from 100 μL urine, diluted with 25 μL internal standard solution and 175 μL 130 mM formic acid, across a supported liquid membrane (SLM) consisting of 9 μL of a 1:1(w/w) mixture of 2-nitrophenyloctyl ether (NPOE) and bis(2-ethylhexyl)phosphite (DEHPi) into an acceptor phase containing 300 μL 130 mM formic acid. The extraction was facilitated by the application of 30 V for 15 min. Enantiomeric separation was achieved using UHPSFC-MS/MS with a chiral stationary phase. The calibration range was 50–10,000 ng/mL for each enantiomer. The between-assay CV was ≤5%, within-assay CV ≤ 1.5%, and bias within ±2%. Recoveries were 83%–90% (CV ≤ 6%), and internal standard corrected matrix effects were 99–105 (CV ≤ 2%). The matrix effects ranged from 96% to 98% (CV ≤ 8%) when not corrected by the internal standard. The EME method was compared with a chiral routine method that employed liquid–liquid extraction (LLE) for sample preparation. Assay results were in agreement with the routine method, and the mean deviation between methods was 3%, ranging from −21% to 31%. Finally, sample preparation greenness was assessed using the AGREEprep tool, which resulted in a greenness score of 0.54 for conductive vial EME, opposed to 0.47 for semi-automated 96-well LLE.
中文翻译:

导电小瓶电膜萃取超高效超临界流体色谱串联质谱法测定尿液中的苯丙胺对映体
安非他明对映体的分离和定量通常用于区分处方安非他明(主要是S-安非他明)和非法形式的药物(外消旋体)的消耗。在本研究中,将原型导电瓶的电膜萃取与超高性能超临界流体色谱 (UHPSFC-MS/MS) 相结合,以定量尿液中的R - 和S - 安非他明。从 100 μL 尿液中提取苯丙胺,用 25 μL 内标溶液和 175 μL 130 mM 甲酸稀释,穿过由 9 μL 1:1(w/w)2-硝基苯基辛基混合物组成的支持液膜 (SLM)乙醚 (NPOE) 和双(2-乙基己基)亚磷酸酯 (DEHPi) 进入含有 300 μL 130 mM 甲酸的受体相。施加 30 V 电压 15 分钟以促进提取。使用 UHPSFC-MS/MS 和手性固定相实现对映体分离。每种对映体的校准范围为 50–10,000 ng/mL。测定间 CV ≤ 5%,测定内 CV ≤ 1.5%,偏差在 ±2% 以内。回收率为 83%–90% (CV ≤ 6%),内标校正基质效应为 99–105 (CV ≤ 2%)。未经内标校正时,基质效应范围为 96% 至 98% (CV ≤ 8%)。将 EME 方法与采用液液萃取 (LLE) 进行样品制备的手性常规方法进行比较。测定结果与常规方法一致,方法间平均偏差为3%,范围为-21%至31%。最后,使用 AGREEprep 工具评估样品制备的绿色度,导电瓶 EME 的绿色度得分为 0.54,而半自动 96 孔 LLE 的绿色度得分为 0.47。
更新日期:2023-04-28
中文翻译:

导电小瓶电膜萃取超高效超临界流体色谱串联质谱法测定尿液中的苯丙胺对映体
安非他明对映体的分离和定量通常用于区分处方安非他明(主要是S-安非他明)和非法形式的药物(外消旋体)的消耗。在本研究中,将原型导电瓶的电膜萃取与超高性能超临界流体色谱 (UHPSFC-MS/MS) 相结合,以定量尿液中的R - 和S - 安非他明。从 100 μL 尿液中提取苯丙胺,用 25 μL 内标溶液和 175 μL 130 mM 甲酸稀释,穿过由 9 μL 1:1(w/w)2-硝基苯基辛基混合物组成的支持液膜 (SLM)乙醚 (NPOE) 和双(2-乙基己基)亚磷酸酯 (DEHPi) 进入含有 300 μL 130 mM 甲酸的受体相。施加 30 V 电压 15 分钟以促进提取。使用 UHPSFC-MS/MS 和手性固定相实现对映体分离。每种对映体的校准范围为 50–10,000 ng/mL。测定间 CV ≤ 5%,测定内 CV ≤ 1.5%,偏差在 ±2% 以内。回收率为 83%–90% (CV ≤ 6%),内标校正基质效应为 99–105 (CV ≤ 2%)。未经内标校正时,基质效应范围为 96% 至 98% (CV ≤ 8%)。将 EME 方法与采用液液萃取 (LLE) 进行样品制备的手性常规方法进行比较。测定结果与常规方法一致,方法间平均偏差为3%,范围为-21%至31%。最后,使用 AGREEprep 工具评估样品制备的绿色度,导电瓶 EME 的绿色度得分为 0.54,而半自动 96 孔 LLE 的绿色度得分为 0.47。

































 京公网安备 11010802027423号
京公网安备 11010802027423号