当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Free Amino Group Transfer via α-Amination of Native Carbonyls
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2023-04-28 , DOI: 10.1002/anie.202304990
Minghao Feng 1 , Anthony J Fernandes 1, 2 , Ana Sirvent 1, 2 , Eleonora Spinozzi 1 , Saad Shaaban 1 , Nuno Maulide 1, 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2023-04-28 , DOI: 10.1002/anie.202304990
Minghao Feng 1 , Anthony J Fernandes 1, 2 , Ana Sirvent 1, 2 , Eleonora Spinozzi 1 , Saad Shaaban 1 , Nuno Maulide 1, 2
Affiliation
|
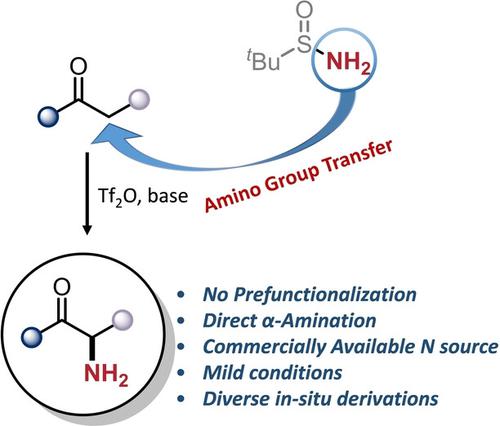
An amino group transfer strategy was developed to install free NH2 to the α-position of native carbonyls under mild conditions. The resulting primary α-aminated products are amenable to in situ peptide coupling or Pictet–Spengler cyclization in one-pot procedures. We anticipate this method to provide a general platform towards the synthesis of amines, ultimately opening exciting avenues in the synthesis and medicinal study of peptidomimetics.
中文翻译:

通过天然羰基的 α-氨基化进行游离氨基转移
开发了氨基转移策略以在温和条件下将游离NH 2安装到天然羰基的α位。所得初级 α-胺化产物适合在一锅程序中进行原位肽偶联或 Pictet-Spengler 环化。我们预计这种方法将为胺的合成提供一个通用平台,最终为肽模拟物的合成和医学研究开辟令人兴奋的途径。
更新日期:2023-04-28
中文翻译:

通过天然羰基的 α-氨基化进行游离氨基转移
开发了氨基转移策略以在温和条件下将游离NH 2安装到天然羰基的α位。所得初级 α-胺化产物适合在一锅程序中进行原位肽偶联或 Pictet-Spengler 环化。我们预计这种方法将为胺的合成提供一个通用平台,最终为肽模拟物的合成和医学研究开辟令人兴奋的途径。

































 京公网安备 11010802027423号
京公网安备 11010802027423号