Clinical and Experimental Nephrology ( IF 2.2 ) Pub Date : 2023-04-28 , DOI: 10.1007/s10157-023-02351-z Yani Yu 1 , Lingyu Xu 1 , Ting Xu 2 , Chengyu Yang 1 , Quandong Bu 1 , Wei Zhang 1 , Long Zhao 1 , Yan Xu 1 , Wei Jiang 1
|
Background
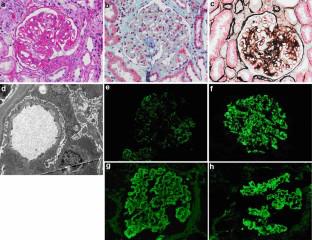
HBV-GN is one of the most common secondary kidney diseases in China. Entecavir is a first-line antiviral therapy in patients with HBV-GN.
Objective
This retrospective study explored whether entecavir is effective and safe for the treatment of HBV-GN with renal insufficiency.
Methods
We screened patients diagnosed with HBV-GN in The Affiliated Hospital of Qingdao University who had elevated serum creatinine levels. Group 1 (30 patients) was given entecavir as antiviral treatment. Group 2 (28 patients) was treated with ARBs. Changes in renal function and the possible influencing factors were observed, with a mean follow-up duration of 36 months.
Results
At the end of follow-up, the elevation in the serum creatinine level and reduction in the eGFR were greater in group 1 than in group 2. The overall renal survival rate, using eGFR < 15 ml/min as the primary renal end point, was 96.7% in group 1 and 67.9% in group 2. Urine protein excretion was decreased in both groups. Treatment with entecavir and the remission of proteinuria were protective factors against renal function impairment, while a lower baseline eGFR was a risk factor for progression to ESRD.
Conclusions
Entecavir slows the progression of renal function impairment in HBV-GN and exerts a significant renal protective effect.




















































 京公网安备 11010802027423号
京公网安备 11010802027423号