当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Investigating the Electrochemically Driven Capture and Release of Long-Chain PFAS by Redox Metallopolymer Sorbents
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2023-04-28 , DOI: 10.1021/acsami.3c01670 Paola Baldaguez Medina 1 , Valentina Ardila Contreras 1 , Frank Hartmann 2 , Deborah Schmitt 2 , Angelique Klimek 1 , Johannes Elbert 1 , Markus Gallei 2, 3 , Xiao Su 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2023-04-28 , DOI: 10.1021/acsami.3c01670 Paola Baldaguez Medina 1 , Valentina Ardila Contreras 1 , Frank Hartmann 2 , Deborah Schmitt 2 , Angelique Klimek 1 , Johannes Elbert 1 , Markus Gallei 2, 3 , Xiao Su 1
Affiliation
|
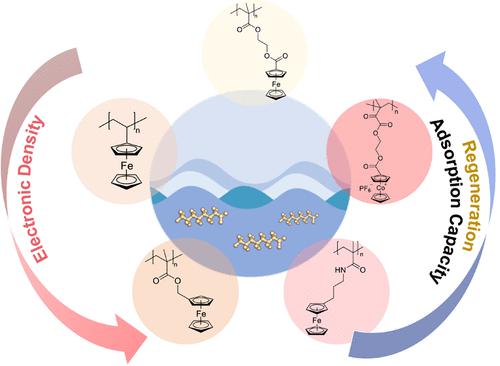
The remediation of perfluoroalkyl substances (PFAS) is an urgent challenge due to their prevalence and persistence in the environment. Electrosorption is a promising approach for wastewater treatment and water purification, especially through the use of redox polymers to control the binding and release of target contaminants without additional external chemical inputs. However, the design of efficient redox electrosorbents for PFAS faces the significant challenge of balancing a high adsorption capacity while maintaining significant electrochemical regeneration. To overcome this challenge, we investigate redox-active metallopolymers as a versatile synthetic platform to enhance both electrochemical reversibility and electrosorption uptake capacity for PFAS removal. We selected and synthesized a series of metallopolymers bearing ferrocene and cobaltocenium units spanning a range of redox potentials to evaluate their performance for the capture and release of perfluorooctanoic acid (PFOA). Our results demonstrate that PFOA uptake and regeneration efficiency increased with more negative formal potential of the redox polymers, indicating possible structural correlations with the electron density of the metallocenes. Poly(2-(methacryloyloxy)ethyl cobaltoceniumcarboxylate hexafluorophosphate) (PMAECoPF6) showed the highest affinity toward PFOA, with an uptake capacity of more than 90 mg PFOA/g adsorbent at 0.0 V vs Ag/AgCl and a regeneration efficiency of more than 85% at −0.4 V vs Ag/AgCl. Kinetics of PFOA release showed that electrochemical bias greatly enhanced the regeneration efficiency when compared to open-circuit desorption. In addition, electrosorption of PFAS from different wastewater matrices and a range of salt concentrations demonstrated the capability of PFAS remediation in complex water sources, even at ppb levels of contaminants. Our work showcases the synthetic tunability of redox metallopolymers for enhanced electrosorption capacity and regeneration of PFAS.
中文翻译:

研究氧化还原金属聚合物吸附剂对长链 PFAS 的电化学驱动捕获和释放
全氟烷基物质 (PFAS) 的修复是一项紧迫的挑战,因为它们在环境中普遍存在且持久存在。电吸附是一种很有前途的废水处理和水净化方法,特别是通过使用氧化还原聚合物来控制目标污染物的结合和释放,而无需额外的外部化学输入。然而,针对 PFAS 的高效氧化还原电吸附剂的设计面临着平衡高吸附容量和保持显着电化学再生的重大挑战。为了克服这一挑战,我们研究了氧化还原活性金属聚合物作为多功能合成平台,以增强电化学可逆性和电吸附吸收能力以去除 PFAS。我们选择并合成了一系列具有氧化还原电位范围的二茂铁和钴茂单元的金属聚合物,以评估它们捕获和释放全氟辛酸 (PFOA) 的性能。我们的结果表明,PFOA 的吸收和再生效率随着氧化还原聚合物的负形式电位增加而增加,表明可能与茂金属的电子密度存在结构相关性。聚(2-(甲基丙烯酰氧基)乙基钴茂羧酸盐六氟磷酸盐)(PMAECoPF 表明与茂金属的电子密度可能存在结构相关性。聚(2-(甲基丙烯酰氧基)乙基钴茂羧酸盐六氟磷酸盐)(PMAECoPF 表明与茂金属的电子密度可能存在结构相关性。聚(2-(甲基丙烯酰氧基)乙基钴茂羧酸盐六氟磷酸盐)(PMAECoPF6 ) 显示出对 PFOA 的最高亲和力,在 0.0 V vs Ag/AgCl 时的吸收能力超过 90 mg PFOA/g 吸附剂,在-0.4 V vs Ag/AgCl 时的再生效率超过 85%。PFOA 释放动力学表明,与开路解吸相比,电化学偏压大大提高了再生效率。此外,来自不同废水基质和一系列盐浓度的 PFAS 的电吸附证明了 PFAS 在复杂水源中的修复能力,即使是在 ppb 级的污染物。我们的工作展示了氧化还原金属聚合物的合成可调性,以增强 PFAS 的电吸附能力和再生。
更新日期:2023-04-28
中文翻译:

研究氧化还原金属聚合物吸附剂对长链 PFAS 的电化学驱动捕获和释放
全氟烷基物质 (PFAS) 的修复是一项紧迫的挑战,因为它们在环境中普遍存在且持久存在。电吸附是一种很有前途的废水处理和水净化方法,特别是通过使用氧化还原聚合物来控制目标污染物的结合和释放,而无需额外的外部化学输入。然而,针对 PFAS 的高效氧化还原电吸附剂的设计面临着平衡高吸附容量和保持显着电化学再生的重大挑战。为了克服这一挑战,我们研究了氧化还原活性金属聚合物作为多功能合成平台,以增强电化学可逆性和电吸附吸收能力以去除 PFAS。我们选择并合成了一系列具有氧化还原电位范围的二茂铁和钴茂单元的金属聚合物,以评估它们捕获和释放全氟辛酸 (PFOA) 的性能。我们的结果表明,PFOA 的吸收和再生效率随着氧化还原聚合物的负形式电位增加而增加,表明可能与茂金属的电子密度存在结构相关性。聚(2-(甲基丙烯酰氧基)乙基钴茂羧酸盐六氟磷酸盐)(PMAECoPF 表明与茂金属的电子密度可能存在结构相关性。聚(2-(甲基丙烯酰氧基)乙基钴茂羧酸盐六氟磷酸盐)(PMAECoPF 表明与茂金属的电子密度可能存在结构相关性。聚(2-(甲基丙烯酰氧基)乙基钴茂羧酸盐六氟磷酸盐)(PMAECoPF6 ) 显示出对 PFOA 的最高亲和力,在 0.0 V vs Ag/AgCl 时的吸收能力超过 90 mg PFOA/g 吸附剂,在-0.4 V vs Ag/AgCl 时的再生效率超过 85%。PFOA 释放动力学表明,与开路解吸相比,电化学偏压大大提高了再生效率。此外,来自不同废水基质和一系列盐浓度的 PFAS 的电吸附证明了 PFAS 在复杂水源中的修复能力,即使是在 ppb 级的污染物。我们的工作展示了氧化还原金属聚合物的合成可调性,以增强 PFAS 的电吸附能力和再生。































 京公网安备 11010802027423号
京公网安备 11010802027423号