当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visible light-induced synthesis of 1,3-disubstituted bicyclo[1.1.1]pentane ketones via cooperative photoredox and N-heterocyclic carbene catalysis
Green Chemistry ( IF 9.3 ) Pub Date : 2023-04-28 , DOI: 10.1039/d3gc00643c Yan Gao 1 , Zicong Zheng 1 , Yu Zhu 1 , Wenhao Xu 1 , Yu Zhou 1 , Chuanming Yu 1 , Xinpeng Jiang 1
Green Chemistry ( IF 9.3 ) Pub Date : 2023-04-28 , DOI: 10.1039/d3gc00643c Yan Gao 1 , Zicong Zheng 1 , Yu Zhu 1 , Wenhao Xu 1 , Yu Zhou 1 , Chuanming Yu 1 , Xinpeng Jiang 1
Affiliation
|
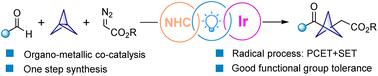
While 1,3-disubstituted bicyclo[1.1.1]pentanes (BCPs) represent an important class of bioisosteres with para-substituted aromatic rings, synthetic methods to access 1,3-disubstituted BCP ketones are still scarce throughout the literature. Herein, we report a cooperative N-heterocyclic carbene (NHC) and iridium dual-catalyzed three-component reaction involving radicals derived from diazo esters to perform an addition reaction onto [1.1.1]propellane to afford BCP radicals. Cross-coupling with a corresponding Breslow intermediate radical then forms a disubstituted BCP ketone. Mild conditions, operational simplicity, and high atom economy render this method a powerful strategy to construct 1,3-disubstituted-BCP ketones from readily available starting materials and with suitable functional group tolerance.
中文翻译:

可见光诱导合成 1,3-二取代双环 [1.1.1] 戊烷酮通过协同光氧化还原和 N-杂环卡宾催化
虽然 1,3-二取代双环[1.1.1] 戊烷 (BCP) 代表了一类重要的具有对位取代芳环的生物等排体,但在整个文献中仍然缺乏获得 1,3-二取代 BCP 酮的合成方法。在此,我们报道了一种合作的 N-杂环卡宾 (NHC) 和铱双催化三组分反应,涉及衍生自重氮酯的自由基在 [1.1.1] 丙烷上进行加成反应以提供 BCP 自由基。然后与相应的 Breslow 中间基交叉偶联形成二取代的 BCP 酮。温和的条件、操作简单和高原子经济性使该方法成为一种强大的策略,可以从易于获得的起始材料中构建 1,3-二取代-BCP 酮,并具有合适的官能团耐受性。
更新日期:2023-04-28
中文翻译:

可见光诱导合成 1,3-二取代双环 [1.1.1] 戊烷酮通过协同光氧化还原和 N-杂环卡宾催化
虽然 1,3-二取代双环[1.1.1] 戊烷 (BCP) 代表了一类重要的具有对位取代芳环的生物等排体,但在整个文献中仍然缺乏获得 1,3-二取代 BCP 酮的合成方法。在此,我们报道了一种合作的 N-杂环卡宾 (NHC) 和铱双催化三组分反应,涉及衍生自重氮酯的自由基在 [1.1.1] 丙烷上进行加成反应以提供 BCP 自由基。然后与相应的 Breslow 中间基交叉偶联形成二取代的 BCP 酮。温和的条件、操作简单和高原子经济性使该方法成为一种强大的策略,可以从易于获得的起始材料中构建 1,3-二取代-BCP 酮,并具有合适的官能团耐受性。































 京公网安备 11010802027423号
京公网安备 11010802027423号