Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2023-04-27 , DOI: 10.1016/j.molstruc.2023.135657 Rahul Kadu , Komal Kolte , Chirag Savani , Sanjio S. Zade , Anvarhusein A. Isab , Atresh Kumar Singh , Vinay K. Singh
|
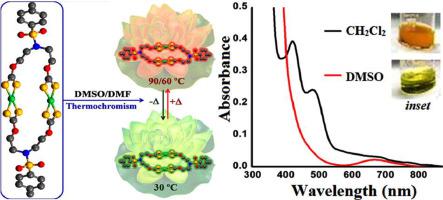
Potassium salt of N,N-Bis(2-dithiocarbonatoethyl)-4-methylbenzenesulfon amide (K2xan) was synthesized and characterized prior to use in the progress of a 24-membered binuclear metallomacrocyclic compound [NiII2-µ2-bis-{(κ2S,S-S2COCH2CH2)2N(Ts)}] (1) and a synthetically challenging 16-member organic functional macrocycle 6,14-ditosyl-1,3,9,11-tetraoxa-6,14-diaza-cyclohexadecane-2,10-dithione (2). Compounds have been characterized by using microanalysis, standard spectroscopic and crystallographic techniques. A plausible mechanism for the formation of compound 2 has been established based on experimental evidence. Compounds 1 and 2 fluoresces at 377 and 293 nm upon excitation at 314 and 232 nm, respectively. The single crystal X-ray diffraction (SCXRD) study revealed a distorted square planar coordination geometry around NiII-centre in 1 which is consistent in the solution of non-coordinating solvents such as CH2Cl2. In a coordinating solvent such as DMSO, however, charge transfer bands 421, 481 nm vanished and characteristic d-d transition band at 698 nm appeared which suggest a change in the coordination geometry around NiII centre from square planar (in CH2Cl2) to octahedral complex (in DMSO). This solvatochromic behaviour of NiII-xanthate complex 1 is supplemented by reversible thermochromic behaviour over a wide temperature ca 30–90°C. Notably, thermal degradation of NiII-xanthate complex 1 gave a stable residual mass that corresponds to NiS. The X-band EPR, UV–visible and TD-DFT studies and electrochemical investigations have been performed to rationalize the results and establish the structure-property correlation.
中文翻译:

大环 NiII-黄药 [NiII2-µ2-bis-{(κ2S,S-S2COCH2CH2)2N(Ts)}] 络合物和环状硫代碳酸酯单体:合成、结晶学、光物理、TD-DFT 和热致变色现象研究
在用于 24 元双核金属大环化合物[ Ni II 2 - µ 2 -bis -{( κ 2 S,S -S 2 COCH 2 CH 2 ) 2N(Ts)}] (1) 和具有综合挑战性的 16 元有机功能大环化合物 6,14-ditosyl-1,3,9,11-tetraoxa-6,14-diaza-cyclohexadecane-2,10-dithione (2 ). 化合物已通过使用微量分析、标准光谱和晶体学技术进行表征。已根据实验证据建立了化合物 2 形成的合理机制。化合物 1 和 2 在 314 和 232 nm 激发后分别在 377 和 293 nm 发出荧光。单晶 X 射线衍射 (SCXRD) 研究揭示了 1 中 Ni II中心周围扭曲的正方形平面配位几何结构,这在非配位溶剂(例如 CH 2 Cl 2 )溶液中是一致的. 然而,在配位溶剂(例如 DMSO)中,电荷转移带 421、481 nm 消失,并且出现 698 nm 处的特征 dd 过渡带,这表明 Ni II中心周围的配位几何形状从方形平面(在 CH 2 Cl 2中)到八面体复合物(在 DMSO 中)。Ni II -黄药络合物 1的这种溶剂化变色行为在ca 30–90°C 的宽温度范围内通过可逆热致变色行为得到补充。值得注意的是,Ni II的热降解-黄药络合物 1 给出了对应于 NiS 的稳定残余质量。已经进行了 X 波段 EPR、紫外-可见和 TD-DFT 研究以及电化学研究以使结果合理化并建立结构-性能相关性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号