Carbon ( IF 10.5 ) Pub Date : 2023-04-27 , DOI: 10.1016/j.carbon.2023.118052 Tong Song , Xiao Zhang , Cong Xie , Ping Yang
|
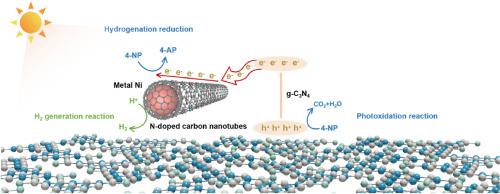
Carbon nanotubes with well conductivity can improve photogenerated carrier separation and transport for enhanced photocatalytic performance. In this paper, Ni nanoparticles embedded N-doped carbon nanotubes (CNTs) were decorated on superior thin g-C3N4 nanosheets as a co-catalyst via a mechano-chemical pre-reaction and thermal polymerization at high temperature. The admirable conductivity and unique one-dimensional structure of Ni-decorated CNTs (NiCNTs) generated more active sites and supplied efficient charge transfer. Abundant unpaired electron and π-conjugated structure of N-doped CNTs promoted the delocalization, retarded recombination, and stabilized charge separation. Ni nanoparticles acted as active sites to trap photogenerated electrons for photoreduction reaction. g-C3N4/NiCNTs composites with an optimized ratio revealed a hydrogen (H2) generation rate as high as 1050.4 μmol g−1 h−1, exhibiting an increase of ∼137 times compared with pure g-C3N4 without using Pt as co-catalyst. The H2 generation rate was even higher than that of g-C3N4/Pt, suggesting that NiCNTs can substitute noble metals completely. In addition, the g-C3N4/NiCNTs sample removed 4-nitrophenol (4-NP) within 10 min under visible light irradiation. The rate constant of g-C3N4/NiCNTs sample was ∼49 times of that of pristine g-C3N4. This work proposed a novel bifunctional catalyst for pollutant treatment and clean energy production.
中文翻译:

N 掺杂碳纳米管增强 Ni 纳米颗粒和 g-C3N4 纳米片之间的电荷传输,用于光催化 H2 生成和 4-硝基苯酚去除
具有良好导电性的碳纳米管可以改善光生载流子的分离和传输,从而提高光催化性能。在本文中,通过机械化学预反应和高温热聚合,将嵌入 N 掺杂碳纳米管 (CNT) 的 Ni 纳米粒子作为助催化剂装饰在超薄 gC 3 N 4纳米片上。Ni-decorated CNTs (NiCNTs) 优异的导电性和独特的一维结构产生了更多的活性位点并提供了有效的电荷转移。N 掺杂 CNT 丰富的未成对电子和 π 共轭结构促进了离域化、延迟复合和稳定的电荷分离。Ni 纳米粒子充当活性位点以捕获光生电子以进行光还原反应。GC具有优化比例的3 N 4 /NiCNTs 复合材料显示出高达 1050.4 μmol g −1 h −1的氢 (H 2 ) 生成速率,与未使用 Pt 作为 co 的纯 gC 3 N 4相比增加了~137倍-催化剂。H 2生成率甚至高于gC 3 N 4 /Pt,表明NiCNTs可以完全替代贵金属。此外,gC 3 N 4 /NiCNTs样品在可见光照射下可在10分钟内去除4-硝基苯酚(4-NP)。gC 3 N 4的速率常数/NiCNTs 样品是原始 gC 3 N 4的~49 倍。这项工作提出了一种用于污染物处理和清洁能源生产的新型双功能催化剂。































 京公网安备 11010802027423号
京公网安备 11010802027423号