Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interfacial Synergy between the Cu Atomic Layer and CeO2 Promotes CO Electrocoupling to Acetate
ACS Nano ( IF 15.8 ) Pub Date : 2023-04-27 , DOI: 10.1021/acsnano.3c00817 Tang Yang, Li Lin, Ximeng Lv, Hongcen Yang, Huishu Feng, Zhongliang Huang, Jiwei Li, Chih-Wen Pao, Zhiwei Hu, Changhong Zhan, Yong Xu, Lan-Sun Zheng, Feng Jiao, Xiaoqing Huang
ACS Nano ( IF 15.8 ) Pub Date : 2023-04-27 , DOI: 10.1021/acsnano.3c00817 Tang Yang, Li Lin, Ximeng Lv, Hongcen Yang, Huishu Feng, Zhongliang Huang, Jiwei Li, Chih-Wen Pao, Zhiwei Hu, Changhong Zhan, Yong Xu, Lan-Sun Zheng, Feng Jiao, Xiaoqing Huang
|
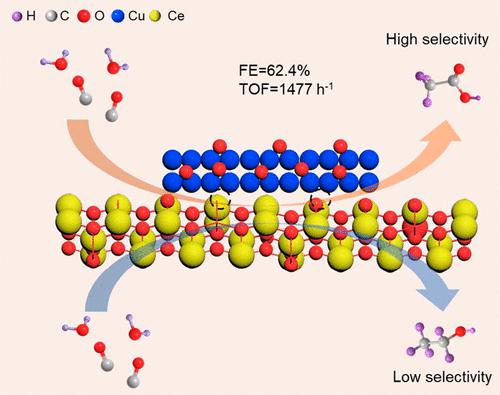
Cu is considered to be an effective electrocatalyst in CO/CO2 reduction reactions (CORR/CO2RR) because of its C–C coupling into C2+ products, but it still remains a formidable challenge to rationally design Cu-based catalysts for highly selective CO/CO2 reduction to C2+ liquid products such as acetate. We here demonstrate that spraying atomically layered Cu atoms onto CeO2 nanorods (Cu–CeO2) can lead to a catalyst with an enhanced acetate selectivity in CORR. Owing to the existence of oxygen vacancies (Ov) in CeO2, the layer of Cu atoms at interface coordinates with Ce atoms in the form of Cu–Ce (Ov), as a result of strong interfacial synergy. The Cu–Ce (Ov) significantly promotes the adsorption and dissociation of H2O, which further couples with CO to selectively produce acetate as the dominant liquid product. In the current density range of 50–150 mA cm–2, the Faradaic efficiencies (FEs) of acetate are over 50% with a maximum value of 62.4%. In particular, the turnover frequency of Cu–CeO2 reaches 1477 h–1, surpassing that of Cu nanoparticle-decorated CeO2 nanorods, bare CeO2 nanorods, as well as other existing Cu-based catalysts. This work advances the rational design of high-performance catalysts for CORR to highly value-added products, which may attract great interests in diverse fields including materials science, chemistry, and catalysis.
中文翻译:

Cu 原子层与 CeO2 之间的界面协同作用促进 CO 电偶联至乙酸盐
Cu 被认为是 CO/CO 2还原反应 (CORR/CO 2 RR) 中的一种有效电催化剂,因为它的 C–C 偶联成 C 2+产物,但合理设计 Cu 基催化剂仍然是一个艰巨的挑战高选择性 CO/CO 2还原成 C 2+液体产品,例如醋酸盐。我们在这里证明,将原子层状铜原子喷涂到 CeO 2纳米棒 (Cu–CeO 2 ) 上可以产生一种催化剂,该催化剂在 CO2RR 中具有增强的乙酸盐选择性。由于CeO 2中氧空位(O v )的存在,界面层Cu原子与Ce原子以Cu-Ce (Ov ), 作为强大的界面协同作用的结果。Cu-Ce (O v ) 显着促进了 H 2 O的吸附和解离,H 2 O 进一步与 CO 结合,选择性地产生乙酸盐作为主要液体产物。在 50–150 mA cm –2的电流密度范围内,醋酸盐的法拉第效率 (FEs) 超过 50%,最大值为 62.4%。特别是,Cu-CeO 2的周转频率达到1477 h –1,超过了Cu纳米颗粒装饰的CeO 2纳米棒、裸CeO 2的周转频率纳米棒,以及其他现有的铜基催化剂。这项工作将用于 CO2RR 的高性能催化剂的合理设计推进到高附加值的产品,这可能会引起包括材料科学、化学和催化在内的多个领域的极大兴趣。
更新日期:2023-04-27
中文翻译:

Cu 原子层与 CeO2 之间的界面协同作用促进 CO 电偶联至乙酸盐
Cu 被认为是 CO/CO 2还原反应 (CORR/CO 2 RR) 中的一种有效电催化剂,因为它的 C–C 偶联成 C 2+产物,但合理设计 Cu 基催化剂仍然是一个艰巨的挑战高选择性 CO/CO 2还原成 C 2+液体产品,例如醋酸盐。我们在这里证明,将原子层状铜原子喷涂到 CeO 2纳米棒 (Cu–CeO 2 ) 上可以产生一种催化剂,该催化剂在 CO2RR 中具有增强的乙酸盐选择性。由于CeO 2中氧空位(O v )的存在,界面层Cu原子与Ce原子以Cu-Ce (Ov ), 作为强大的界面协同作用的结果。Cu-Ce (O v ) 显着促进了 H 2 O的吸附和解离,H 2 O 进一步与 CO 结合,选择性地产生乙酸盐作为主要液体产物。在 50–150 mA cm –2的电流密度范围内,醋酸盐的法拉第效率 (FEs) 超过 50%,最大值为 62.4%。特别是,Cu-CeO 2的周转频率达到1477 h –1,超过了Cu纳米颗粒装饰的CeO 2纳米棒、裸CeO 2的周转频率纳米棒,以及其他现有的铜基催化剂。这项工作将用于 CO2RR 的高性能催化剂的合理设计推进到高附加值的产品,这可能会引起包括材料科学、化学和催化在内的多个领域的极大兴趣。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号