当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Redox-Neutral Intramolecular Dearomative Spirocyclization of Phenols Induced by Visible Light
Organic Letters ( IF 4.9 ) Pub Date : 2023-04-26 , DOI: 10.1021/acs.orglett.3c01257
Linlin Zhang 1 , Fengchi Hu 1 , Lei Shen 1 , Lijuan Gao 1 , Yunhong Yang 1 , Zhiqiang Pan 1 , Chengfeng Xia 1
Organic Letters ( IF 4.9 ) Pub Date : 2023-04-26 , DOI: 10.1021/acs.orglett.3c01257
Linlin Zhang 1 , Fengchi Hu 1 , Lei Shen 1 , Lijuan Gao 1 , Yunhong Yang 1 , Zhiqiang Pan 1 , Chengfeng Xia 1
Affiliation
|
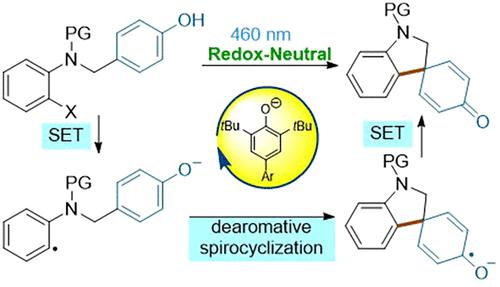
Described herein is a redox-neutral intramolecular dearomative spirocyclization induced by visible light. The photochemical cyclization was catalyzed by a phenolate anion-derived photocatalyst and delivered the spirocyclohexadienone. Mechanistic experiments revealed that the aryl halide was reduced to aryl radical via the single-electron transfer (SET) process under visible light irradiation. The electrophilic addition of an aryl radical with the phenolate anion moiety gave a radical anion intermediate, which recycled the photocatalyst by a second SET process.
中文翻译:

可见光诱导的酚类氧化还原中性分子内脱芳螺环化
本文描述的是由可见光诱导的氧化还原中性分子内脱芳环化。光化学环化由酚盐阴离子衍生的光催化剂催化,并产生螺环己二烯酮。机理实验表明,芳基卤化物在可见光照射下通过单电子转移 (SET) 过程还原为芳基自由基。芳基与酚盐阴离子部分的亲电加成产生自由基阴离子中间体,通过第二个 SET 过程回收光催化剂。
更新日期:2023-04-26
中文翻译:

可见光诱导的酚类氧化还原中性分子内脱芳螺环化
本文描述的是由可见光诱导的氧化还原中性分子内脱芳环化。光化学环化由酚盐阴离子衍生的光催化剂催化,并产生螺环己二烯酮。机理实验表明,芳基卤化物在可见光照射下通过单电子转移 (SET) 过程还原为芳基自由基。芳基与酚盐阴离子部分的亲电加成产生自由基阴离子中间体,通过第二个 SET 过程回收光催化剂。

































 京公网安备 11010802027423号
京公网安备 11010802027423号