European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2023-04-26 , DOI: 10.1016/j.ejmech.2023.115388 Chuanbiao Du 1 , Xinlong Yang 1 , Yan Long 1 , Xueqing Lang 2 , Lige Liu 1 , Yajie Xu 1 , Hu Wu 1 , Yiwen Chu 3 , Xiaolei Hu 2 , Junfeng Deng 3 , Qinggang Ji 1
|
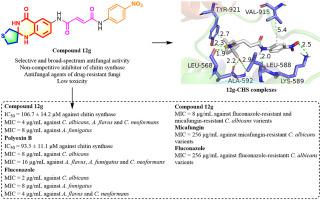
A series of spiro-quinazolinone scaffolds were constructed based on the bioactivity of quinazolinone and the inherent feature of spirocycle to design novel chitin synthase inhibitors that possess mode of action different from that of the currently used antifungal agents. Among them, the spiro[thiophen-quinazolin]-one derivatives containing α, β-unsaturated carbonyl fragments had shown inhibitory activities against chitin synthase and antifungal activities. The enzymatic experiments showed that among the sixteen compounds, compounds 12d, 12g, 12j, 12l and 12m exhibited inhibitions against chitin synthase with IC50 values of 116.7 ± 19.6 μM, 106.7 ± 14.2 μM, 102.3 ± 9.6 μM, 122.7 ± 22.2 μM and 136.8 ± 12.4 μM, respectively, which were comparable to that of polyoxin B (IC50 = 93.5 ± 11.1 μM). The assays of enzymatic Kinetic parameters showed that compound 12g was a non-competitive inhibitor of chitin synthase. The antifungal assays showed that compounds 12d, 12g, 12j, 12l and 12m exhibited a broad-spectrum of antifungal activity against the four strains tested in vitro. In which, compounds 12g and 12j had stronger antifungal activity against four tested strains than that of polyoxin B and similar to that of fluconazole, while compounds 12d, 12l and 12m showed antifungal activity comparable to that of polyoxin B against four tested strains. Meanwhile, compounds 12d, 12g, 12j, 12l and 12m exhibited good antifungal activity against fluconazole-resistant and micafungin-resistant fungi variants with MIC values ranging from 4 to 32 μg/mL while the MIC values of reference drugs were above 256 μg/mL. Furthermore, the results of drug-combination experiments showed that compounds 12d, 12g, 12j, 12l and 12m had synergistic or additive effects with fluconazole or polyoxin B. The results of sorbitol protection experiment and the experiment of antifungal activity against micafungin-resistant fungi further demonstrated that these compounds target chitin synthase. The result of cytotoxicity assay showed that compound 12g had low toxicity toward human lung cancer A549 cells and the ADME analysis in silico displayed that compound 12g possessed promising pharmacokinetic properties. The molecular docking indicated that compound 12g formed multiple hydrogen bond interactions binding to chitin synthase, which might be conductive to increasing the binding affinity and inhibiting the activity of chitin synthase. The above results indicated that the designed compounds were chitin synthase inhibitors with selectivity and broad-spectrum antifungal activity and could be act as the lead compounds against drug-resistant fungi.
中文翻译:

作为几丁质合酶抑制剂和抗真菌剂的新型螺喹唑啉酮衍生物的设计、合成及生物学评价
基于喹唑啉酮的生物活性和螺环的固有特性,构建了一系列螺环喹唑啉酮支架,设计出与目前使用的抗真菌药物作用方式不同的新型几丁质合酶抑制剂。其中,含有α,β-不饱和羰基片段的螺[噻吩-喹唑啉]-酮衍生物显示出对几丁质合酶的抑制活性和抗真菌活性。酶促实验表明,在16个化合物中,化合物12d、12g、12j、12l和12m对几丁质合酶具有抑制作用,IC50值分别为 116.7 ± 19.6 μM、106.7 ± 14.2 μM、102.3 ± 9.6 μM、122.7 ± 22.2 μM 和 136.8 ± 12.4 μM,与多氧菌素 B (IC 50 = 93.5 ± 11.1 μM)相当。酶促动力学参数测定表明化合物12g是几丁质合酶的非竞争性抑制剂。抗真菌试验表明,化合物12d、12g、12j、12l和12m对体外测试的四种菌株表现出广谱抗真菌活性。其中,化合物12g和12j化合物 12d、12l 和 12m 对四种受试菌株的抗真菌活性强于多氧菌素 B,与氟康唑相似,而化合物12d、12l和12m对四种受试菌株的抗真菌活性与多氧菌素 B 相当。同时,化合物12d、12g、12j、12l和12m对耐氟康唑和耐米卡芬净的真菌变体表现出良好的抗真菌活性,MIC值范围为4~32 μg/mL,而参比药物的MIC值均在256 μg/mL以上. 此外,药物组合实验的结果表明,化合物12d , 12g, 12j , 12l和12m与氟康唑或多氧菌素 B 具有协同或相加作用。山梨糖醇保护实验和对米卡芬净耐药真菌的抗真菌活性实验结果进一步证明了这些化合物靶向几丁质合酶。细胞毒性测定结果表明化合物12g对人肺癌A549细胞具有低毒性,计算机ADME分析显示化合物12g具有良好的药代动力学特性。分子对接表明,化合物12g与几丁质合酶形成多个氢键相互作用,可能有助于提高结合亲和力并抑制几丁质合酶的活性。以上结果表明,所设计的化合物是几丁质合酶抑制剂,具有选择性和广谱抗真菌活性,可作为抗耐药真菌的先导化合物。






























 京公网安备 11010802027423号
京公网安备 11010802027423号