当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Near-Zero-Waste Approach for the Treatment of Saprolitic Nickel Laterite via a Combined Hydrometallurgical Process
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2023-04-26 , DOI: 10.1021/acssuschemeng.2c05403
Guanghui Li 1 , Yanhu Chen 1 , Changye Mang 1 , Xin Teng 1 , Jun Luo 2 , Mingjun Rao 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2023-04-26 , DOI: 10.1021/acssuschemeng.2c05403
Guanghui Li 1 , Yanhu Chen 1 , Changye Mang 1 , Xin Teng 1 , Jun Luo 2 , Mingjun Rao 1
Affiliation
|
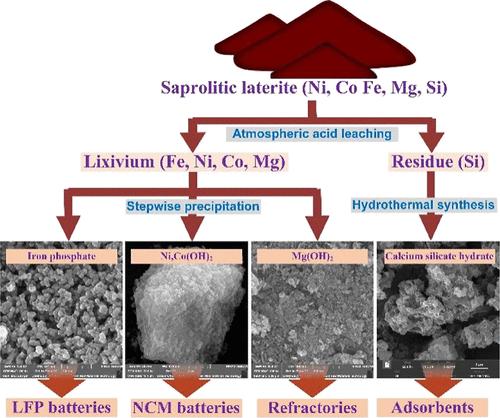
Atmospheric acid leaching can be used for processing the nickel laterite ore. The lixivium composition necessitates Fe3+ separation as the first step in recovering valuable metals. However, traditional partial neutralization has the problem of significant loss of Ni2+ and Co2+ ions due to the high Fe3+ concentration. Besides, the discharged acidic residue leads to environmental pollution. This study proposes a combined hydrometallurgical method for efficiently recovering valuable components from saprolitic laterite. After atmospheric sulfuric acid leaching, the Fe3+ in the lixivium was first separated using phosphoric acid and sodium hydroxide as reagents; 92% of the Fe3+ precipitated as iron phosphate at a pH of 1.8. The charge and discharge capacities of the LFP sample prepared from this iron phosphate at the rate of 1 C were 157 and 154 mA h/g, respectively. During the recovery of Fe3+ ions, approximately 100% of other metals were retained in the aqueous solution. After adjustment of the pH to 6.0, this solution was used to remove impurities, including Al, Mn, Cr, and Fe. Then, more than 98% of Ni and Co were recovered as Ni,Co(OH)2 precipitates at a pH of 8.0–9.0. When the pH was raised to 11.0, over 98% of Mg precipitated as Mg(OH)2. The leach residue with a high silica content was used to prepare calcium silicate hydrate (C–S–H) via a hydrothermal process. The recovery of FePO4, Mg(OH)2, and C–S–H, in addition to Ni,Co(OH)2, made this a value-added process and with a near-zero residue discharge.
中文翻译:

通过联合湿法冶金工艺处理腐泥土镍红土的近零废物方法
常压酸浸可用于处理红土镍矿。浸出液成分需要 Fe 3+分离作为回收有价值金属的第一步。然而,由于Fe 3+浓度高,传统的部分中和存在Ni 2+和Co 2+离子大量损失的问题。此外,排放的酸性残渣会造成环境污染。本研究提出了一种联合湿法冶金方法,可以有效地从腐泥土红土中回收有价值的成分。常压硫酸浸出后,首先以磷酸和氢氧化钠为试剂分离浸出液中的Fe 3+ ;92%的Fe 3+在 pH 值为 1.8 时沉淀为磷酸铁。由该磷酸铁制备的 LFP 样品在 1 C 倍率下的充放电容量分别为 157 和 154 mA h/g。在回收 Fe 3+离子期间,大约 100% 的其他金属保留在水溶液中。将 pH 调节至 6.0 后,使用该溶液去除杂质,包括 Al、Mn、Cr 和 Fe。然后,在 pH 为 8.0–9.0 时,超过 98% 的 Ni 和 Co 以 Ni,Co(OH) 2沉淀物的形式被回收。当 pH 升至 11.0 时,超过 98% 的 Mg 以 Mg(OH) 2 的形式沉淀。将二氧化硅含量高的浸出渣用于通过水热法制备硅酸钙水合物 (C–S–H)。FePO 4、Mg(OH)的回收2和 C–S–H,以及 Ni,Co(OH) 2,使该工艺成为一个增值工艺,并且残留物排放量接近于零。
更新日期:2023-04-26
中文翻译:

通过联合湿法冶金工艺处理腐泥土镍红土的近零废物方法
常压酸浸可用于处理红土镍矿。浸出液成分需要 Fe 3+分离作为回收有价值金属的第一步。然而,由于Fe 3+浓度高,传统的部分中和存在Ni 2+和Co 2+离子大量损失的问题。此外,排放的酸性残渣会造成环境污染。本研究提出了一种联合湿法冶金方法,可以有效地从腐泥土红土中回收有价值的成分。常压硫酸浸出后,首先以磷酸和氢氧化钠为试剂分离浸出液中的Fe 3+ ;92%的Fe 3+在 pH 值为 1.8 时沉淀为磷酸铁。由该磷酸铁制备的 LFP 样品在 1 C 倍率下的充放电容量分别为 157 和 154 mA h/g。在回收 Fe 3+离子期间,大约 100% 的其他金属保留在水溶液中。将 pH 调节至 6.0 后,使用该溶液去除杂质,包括 Al、Mn、Cr 和 Fe。然后,在 pH 为 8.0–9.0 时,超过 98% 的 Ni 和 Co 以 Ni,Co(OH) 2沉淀物的形式被回收。当 pH 升至 11.0 时,超过 98% 的 Mg 以 Mg(OH) 2 的形式沉淀。将二氧化硅含量高的浸出渣用于通过水热法制备硅酸钙水合物 (C–S–H)。FePO 4、Mg(OH)的回收2和 C–S–H,以及 Ni,Co(OH) 2,使该工艺成为一个增值工艺,并且残留物排放量接近于零。

































 京公网安备 11010802027423号
京公网安备 11010802027423号