当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Search of Wasserman’s Catenane
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-04-25 , DOI: 10.1021/jacs.3c01939
Andrei S Baluna 1 , Albano Galan 1 , David A Leigh 1, 2 , Gareth D Smith 1 , Justin T J Spence 1 , Daniel J Tetlow 1 , Iñigo J Vitorica-Yrezabal 1 , Min Zhang 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-04-25 , DOI: 10.1021/jacs.3c01939
Andrei S Baluna 1 , Albano Galan 1 , David A Leigh 1, 2 , Gareth D Smith 1 , Justin T J Spence 1 , Daniel J Tetlow 1 , Iñigo J Vitorica-Yrezabal 1 , Min Zhang 1, 2
Affiliation
|
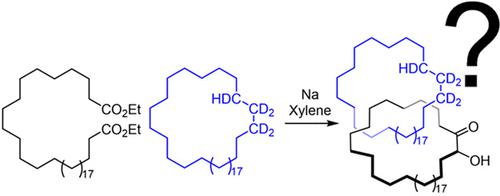
We repeat the earliest claimed [2]catenane synthesis, reported by Wasserman over 60 years ago, in order to ascertain whether or not a nontemplate, statistical synthesis by acyloin macrocyclization does indeed form mechanically interlocked rings. The lack of direct experimental evidence for Wasserman’s catenane has led to it being described as a “prophetic compound”, a technical term used in patents for claimed molecules that have not yet been synthesized. Contemporary synthetic methods were used to reconstruct Wasserman’s deuterium-labeled macrocycle and other building blocks on the 10–100 g reaction scale necessary to generate, in principle, ∼1 mg of catenane. Modern spectrometric and spectroscopic tools and chemical techniques (including tandem mass spectrometry, deuterium nuclear magnetic resonance (NMR) spectroscopy, and fluorescent tag labeling) were brought to bear in an effort to detect, isolate, and prove the structure of a putative [2]catenane consisting of a 34-membered cyclic hydrocarbon mechanically linked with a 34-membered cyclic α-hydroxyketone.
中文翻译:

寻找沃瑟曼的链烷
我们重复了 60 多年前 Wasserman 报道的最早声称的 [2] 链烯合成,以确定非模板、通过偶姻大环化的统计合成是否确实形成机械互锁环。Wasserman 的索烷缺乏直接的实验证据,导致它被描述为“预言性化合物”,这是专利中使用的技术术语,用于指代尚未合成的要求保护的分子。现代合成方法被用于重建 Wasserman 的氘标记的大环化合物和其他结构单元,在 10-100 g 反应规模上,原则上产生约 1 mg 的链烷烃。现代光谱测定和光谱工具以及化学技术(包括串联质谱、氘核磁共振 (NMR) 光谱、
更新日期:2023-04-25
中文翻译:

寻找沃瑟曼的链烷
我们重复了 60 多年前 Wasserman 报道的最早声称的 [2] 链烯合成,以确定非模板、通过偶姻大环化的统计合成是否确实形成机械互锁环。Wasserman 的索烷缺乏直接的实验证据,导致它被描述为“预言性化合物”,这是专利中使用的技术术语,用于指代尚未合成的要求保护的分子。现代合成方法被用于重建 Wasserman 的氘标记的大环化合物和其他结构单元,在 10-100 g 反应规模上,原则上产生约 1 mg 的链烷烃。现代光谱测定和光谱工具以及化学技术(包括串联质谱、氘核磁共振 (NMR) 光谱、







































 京公网安备 11010802027423号
京公网安备 11010802027423号