当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Improving CO2-to-C2+ Product Electroreduction Efficiency via Atomic Lanthanide Dopant-Induced Tensile-Strained CuOx Catalysts
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-04-24 , DOI: 10.1021/jacs.3c02428
Jiaqi Feng 1 , Limin Wu 1, 2 , Shoujie Liu 3 , Liang Xu 1 , Xinning Song 1, 2 , Libing Zhang 1, 2 , Qinggong Zhu 1, 2 , Xinchen Kang 1, 2 , Xiaofu Sun 1, 2 , Buxing Han 1, 2, 4
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-04-24 , DOI: 10.1021/jacs.3c02428
Jiaqi Feng 1 , Limin Wu 1, 2 , Shoujie Liu 3 , Liang Xu 1 , Xinning Song 1, 2 , Libing Zhang 1, 2 , Qinggong Zhu 1, 2 , Xinchen Kang 1, 2 , Xiaofu Sun 1, 2 , Buxing Han 1, 2, 4
Affiliation
|
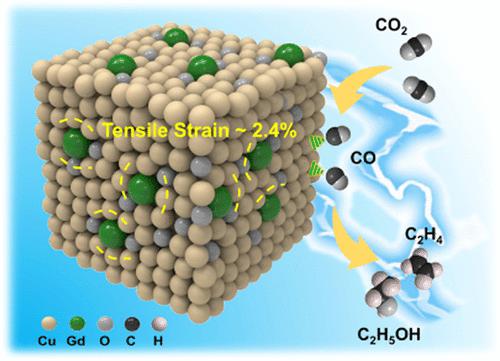
Cu is a promising electrocatalyst in CO2 reduction reaction (CO2RR) to high-value C2+ products. However, as important C–C coupling active sites, the Cu+ species is usually unstable under reduction conditions. How atomic dopants affect the performance of Cu-based catalysts is interesting to be studied. Herein, we first calculated the difference between the thermodynamic limiting potentials of CO2RR and the hydrogen evolution reaction, as well as the *CO binding energy over Cu2O doped with different metals, and the results indicated that doping atomic Gd into Cu2O could improve the performance of the catalyst effectively. On the basis of the theoretical study, we designed Gd1/CuOx catalysts. The distinctive electronic structure and large ion radii of Gd not only keep the Cu+ species stable during the reaction but also induce tensile strain in Gd1/CuOx, resulting in excellent performance of the catalysts for electroreduction of CO2 to C2+ products. The Faradic efficiency of C2+ products could reach 81.4% with a C2+ product partial current density of 444.3 mA cm–2 at −0.8 V vs a reversible hydrogen electrode. Detailed experimental and theoretical studies revealed that Gd doping enhanced CO2 activation on the catalyst, stabilized the key intermediate O*CCO, and reduced the energy barrier of the C–C coupling reaction.
中文翻译:

通过原子镧系掺杂剂诱导的拉伸应变 CuOx 催化剂提高 CO2 到 C2+ 产品的电还原效率
Cu 在 CO 2还原反应 (CO 2 RR) 到高价值 C 2+产品中是一种很有前途的电催化剂。然而,作为重要的 C-C 偶联活性位点,Cu +物种在还原条件下通常不稳定。原子掺杂剂如何影响铜基催化剂的性能值得研究。在此,我们首先计算了CO 2 RR和析氢反应的热力学极限电位的差异,以及不同金属掺杂Cu 2 O上的*CO结合能,结果表明,在Cu 2中掺杂原子GdO可以有效提高催化剂的性能。在理论研究的基础上,我们设计了Gd 1 /CuO x催化剂。Gd 独特的电子结构和大离子半径不仅使 Cu +物种在反应过程中保持稳定,而且在 Gd 1 /CuO x中引起拉伸应变,从而使 CO 2电还原为 C 2+产物的催化剂具有优异的性能. C 2+产品法拉第效率可达81.4%,C 2+产品分电流密度为444.3 mA cm –2在-0.8 V vs 可逆氢电极。详细的实验和理论研究表明,Gd 掺杂增强了催化剂上的CO 2活化,稳定了关键中间体 O*CCO,并降低了 C-C 偶联反应的能垒。
更新日期:2023-04-24
中文翻译:

通过原子镧系掺杂剂诱导的拉伸应变 CuOx 催化剂提高 CO2 到 C2+ 产品的电还原效率
Cu 在 CO 2还原反应 (CO 2 RR) 到高价值 C 2+产品中是一种很有前途的电催化剂。然而,作为重要的 C-C 偶联活性位点,Cu +物种在还原条件下通常不稳定。原子掺杂剂如何影响铜基催化剂的性能值得研究。在此,我们首先计算了CO 2 RR和析氢反应的热力学极限电位的差异,以及不同金属掺杂Cu 2 O上的*CO结合能,结果表明,在Cu 2中掺杂原子GdO可以有效提高催化剂的性能。在理论研究的基础上,我们设计了Gd 1 /CuO x催化剂。Gd 独特的电子结构和大离子半径不仅使 Cu +物种在反应过程中保持稳定,而且在 Gd 1 /CuO x中引起拉伸应变,从而使 CO 2电还原为 C 2+产物的催化剂具有优异的性能. C 2+产品法拉第效率可达81.4%,C 2+产品分电流密度为444.3 mA cm –2在-0.8 V vs 可逆氢电极。详细的实验和理论研究表明,Gd 掺杂增强了催化剂上的CO 2活化,稳定了关键中间体 O*CCO,并降低了 C-C 偶联反应的能垒。

































 京公网安备 11010802027423号
京公网安备 11010802027423号