当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NanoBRET—A Novel BRET Platform for the Analysis of Protein–Protein Interactions
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2015-06-09 00:00:00 , DOI: 10.1021/acschembio.5b00143 Thomas Machleidt 1 , Carolyn C. Woodroofe 2 , Marie K. Schwinn 1 , Jacqui Méndez 1 , Matthew B. Robers 1 , Kris Zimmerman 1 , Paul Otto 1 , Danette L. Daniels 1 , Thomas A. Kirkland 2 , Keith V. Wood 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2015-06-09 00:00:00 , DOI: 10.1021/acschembio.5b00143 Thomas Machleidt 1 , Carolyn C. Woodroofe 2 , Marie K. Schwinn 1 , Jacqui Méndez 1 , Matthew B. Robers 1 , Kris Zimmerman 1 , Paul Otto 1 , Danette L. Daniels 1 , Thomas A. Kirkland 2 , Keith V. Wood 1
Affiliation
|
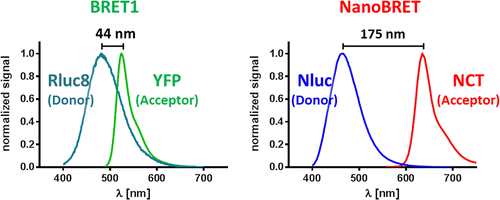
Dynamic interactions between proteins comprise a key mechanism for temporal control of cellular function and thus hold promise for development of novel drug therapies. It remains technically challenging, however, to quantitatively characterize these interactions within the biologically relevant context of living cells. Although, bioluminescence resonance energy transfer (BRET) has often been used for this purpose, its general applicability has been hindered by limited sensitivity and dynamic range. We have addressed this by combining an extremely bright luciferase (Nanoluc) with a means for tagging intracellular proteins with a long-wavelength fluorophore (HaloTag). The small size (19 kDa), high emission intensity, and relatively narrow spectrum (460 nm peak intensity) make Nanoluc luciferase well suited as an energy donor. By selecting an efficient red-emitting fluorophore (635 nm peak intensity) for attachment onto the HaloTag, an overall spectral separation exceeding 175 nm was achieved. This combination of greater light intensity with improved spectral resolution results in substantially increased detection sensitivity and dynamic range over current BRET technologies. Enhanced performance is demonstrated using several established model systems, as well as the ability to image BRET in individual cells. The capabilities are further exhibited in a novel assay developed for analyzing the interactions of bromodomain proteins with chromatin in living cells.
中文翻译:

NanoBRET-用于蛋白质-蛋白质相互作用分析的新型BRET平台
蛋白质之间的动态相互作用是细胞功能暂时控制的关键机制,因此有望开发出新的药物疗法。然而,在活细胞的生物学相关背景下定量表征这些相互作用在技术上仍然具有挑战性。尽管生物发光共振能量转移(BRET)经常用于此目的,但由于灵敏度和动态范围有限,其一般适用性受到了限制。我们已经解决了这一问题,方法是将极亮的荧光素酶(Nanoluc)与用长波长荧光团(HaloTag)标记细胞内蛋白质的方法相结合。小尺寸(19 kDa),高发射强度和相对窄的光谱(460 nm峰值强度)使Nanoluc荧光素酶非常适合作为能量供体。通过选择有效的发红光荧光团(峰值强度为635 nm),将其附着到HaloTag上,可以实现超过175 nm的总光谱分离。与目前的BRET技术相比,更高的光强度与更高的光谱分辨率相结合,可大大提高检测灵敏度和动态范围。使用几个已建立的模型系统以及在单个细胞中对BRET成像的能力,可以证明性能得到了增强。在开发用于分析活细胞中溴结构域蛋白与染色质相互作用的新型测定法中进一步展示了该功能。与目前的BRET技术相比,更高的光强度与更高的光谱分辨率相结合,可显着提高检测灵敏度和动态范围。使用几个已建立的模型系统以及在单个细胞中对BRET成像的能力,可以证明性能得到了增强。在开发用于分析活细胞中溴结构域蛋白与染色质相互作用的新型测定法中进一步展示了该功能。与目前的BRET技术相比,更高的光强度与更高的光谱分辨率相结合,可显着提高检测灵敏度和动态范围。使用几个已建立的模型系统以及在单个细胞中对BRET成像的能力,可以证明性能得到了增强。在开发用于分析活细胞中溴结构域蛋白与染色质相互作用的新型测定法中进一步展示了该功能。
更新日期:2015-06-09
中文翻译:

NanoBRET-用于蛋白质-蛋白质相互作用分析的新型BRET平台
蛋白质之间的动态相互作用是细胞功能暂时控制的关键机制,因此有望开发出新的药物疗法。然而,在活细胞的生物学相关背景下定量表征这些相互作用在技术上仍然具有挑战性。尽管生物发光共振能量转移(BRET)经常用于此目的,但由于灵敏度和动态范围有限,其一般适用性受到了限制。我们已经解决了这一问题,方法是将极亮的荧光素酶(Nanoluc)与用长波长荧光团(HaloTag)标记细胞内蛋白质的方法相结合。小尺寸(19 kDa),高发射强度和相对窄的光谱(460 nm峰值强度)使Nanoluc荧光素酶非常适合作为能量供体。通过选择有效的发红光荧光团(峰值强度为635 nm),将其附着到HaloTag上,可以实现超过175 nm的总光谱分离。与目前的BRET技术相比,更高的光强度与更高的光谱分辨率相结合,可大大提高检测灵敏度和动态范围。使用几个已建立的模型系统以及在单个细胞中对BRET成像的能力,可以证明性能得到了增强。在开发用于分析活细胞中溴结构域蛋白与染色质相互作用的新型测定法中进一步展示了该功能。与目前的BRET技术相比,更高的光强度与更高的光谱分辨率相结合,可显着提高检测灵敏度和动态范围。使用几个已建立的模型系统以及在单个细胞中对BRET成像的能力,可以证明性能得到了增强。在开发用于分析活细胞中溴结构域蛋白与染色质相互作用的新型测定法中进一步展示了该功能。与目前的BRET技术相比,更高的光强度与更高的光谱分辨率相结合,可显着提高检测灵敏度和动态范围。使用几个已建立的模型系统以及在单个细胞中对BRET成像的能力,可以证明性能得到了增强。在开发用于分析活细胞中溴结构域蛋白与染色质相互作用的新型测定法中进一步展示了该功能。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号