当前位置:
X-MOL 学术
›
Mol. Ther. Nucl. Acids
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self-replicating RNA nanoparticle vaccine elicits protective immune responses against SARS-CoV-2
Molecular Therapy - Nucleic Acids ( IF 6.5 ) Pub Date : 2023-04-23 , DOI: 10.1016/j.omtn.2023.04.021 Guibin Lin 1, 2, 3, 4 , Huan Yan 1, 2, 3, 4 , Jing Sun 5 , Jincun Zhao 5, 6 , Yuan Zhang 1, 2, 3, 4
Molecular Therapy - Nucleic Acids ( IF 6.5 ) Pub Date : 2023-04-23 , DOI: 10.1016/j.omtn.2023.04.021 Guibin Lin 1, 2, 3, 4 , Huan Yan 1, 2, 3, 4 , Jing Sun 5 , Jincun Zhao 5, 6 , Yuan Zhang 1, 2, 3, 4
Affiliation
|
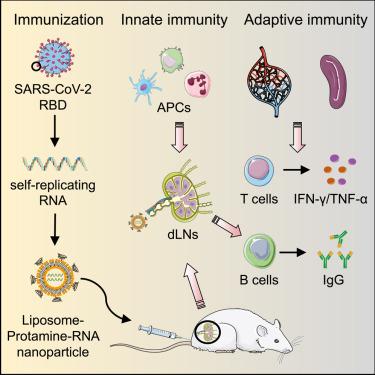
The creation of safe and effective vaccines that induce potent cellular and humoral immune responses against SARS-CoV-2 is urgently needed to end the global COVID-19 epidemic. Here, we developed an alphavirus-derived self-replicating RNA (repRNA)-based vaccine platform encoding the receptor-binding domain (RBD) of SARS-CoV-2 spike glycoprotein. The repRNA triggers prolonged antigen expression compared with conventional mRNA due to the replication machinery of repRNA. To improve the delivery and vaccine efficacy of repRNA, we developed a self-assembling liposome-protamine-RNA (LPR) nanoparticle with highly efficient encapsulation and transfection of repRNA. LPR-repRNA vaccines substantially activated type I interferon response and innate immune signaling pathways. Subcutaneous immunization of LPR-repRNA-RBD led to prolonged antigen expression, stimulation of innate immune cells, and induction of germinal center response in draining lymph nodes. LPR-repRNA-RBD induced antigen-specific T cell responses and skewed cellular immunity toward an effector memory CD8+ T cell response. Immunizations with LPR-repRNA-RBD triggered the production of anti-RBD IgG antibodies and induced neutralizing antibody response against SARS-CoV-2 pseudovirus. LPR-repRNA-RBD vaccines reduced SARS-CoV-2 infection and lung inflammation in mice. Altogether, these data suggest that the LPR-repRNA platform can be a promising avenue for COVID-19 vaccine development.
中文翻译:

自我复制的 RNA 纳米颗粒疫苗引发针对 SARS-CoV-2 的保护性免疫反应
为了结束全球 COVID-19 流行病,迫切需要创造安全有效的疫苗,以诱导针对 SARS-CoV-2 的强效细胞和体液免疫反应。在这里,我们开发了一种基于甲病毒衍生的自复制 RNA (repRNA) 疫苗平台,编码 SARS-CoV-2 刺突糖蛋白的受体结合域 (RBD)。由于 repRNA 的复制机制,与传统 mRNA 相比,repRNA 触发了延长的抗原表达。为了提高 repRNA 的递送和疫苗功效,我们开发了一种自组装脂质体-鱼精蛋白-RNA (LPR) 纳米颗粒,具有高效封装和转染 repRNA。LPR-repRNA 疫苗显着激活了 I 型干扰素反应和先天免疫信号通路。LPR-repRNA-RBD 的皮下免疫导致抗原表达延长、先天免疫细胞刺激和诱导引流淋巴结中的生发中心反应。LPR-repRNA-RBD 诱导抗原特异性 T 细胞反应,并使细胞免疫偏向效应记忆 CD8+ T 细胞反应。用 LPR-repRNA-RBD 免疫触发了抗 RBD IgG 抗体的产生,并诱导了针对 SARS-CoV-2 假病毒的中和抗体反应。LPR-repRNA-RBD 疫苗减少了小鼠的 SARS-CoV-2 感染和肺部炎症。总而言之,这些数据表明 LPR-repRNA 平台可以成为 COVID-19 疫苗开发的有前途的途径。
更新日期:2023-04-23
中文翻译:

自我复制的 RNA 纳米颗粒疫苗引发针对 SARS-CoV-2 的保护性免疫反应
为了结束全球 COVID-19 流行病,迫切需要创造安全有效的疫苗,以诱导针对 SARS-CoV-2 的强效细胞和体液免疫反应。在这里,我们开发了一种基于甲病毒衍生的自复制 RNA (repRNA) 疫苗平台,编码 SARS-CoV-2 刺突糖蛋白的受体结合域 (RBD)。由于 repRNA 的复制机制,与传统 mRNA 相比,repRNA 触发了延长的抗原表达。为了提高 repRNA 的递送和疫苗功效,我们开发了一种自组装脂质体-鱼精蛋白-RNA (LPR) 纳米颗粒,具有高效封装和转染 repRNA。LPR-repRNA 疫苗显着激活了 I 型干扰素反应和先天免疫信号通路。LPR-repRNA-RBD 的皮下免疫导致抗原表达延长、先天免疫细胞刺激和诱导引流淋巴结中的生发中心反应。LPR-repRNA-RBD 诱导抗原特异性 T 细胞反应,并使细胞免疫偏向效应记忆 CD8+ T 细胞反应。用 LPR-repRNA-RBD 免疫触发了抗 RBD IgG 抗体的产生,并诱导了针对 SARS-CoV-2 假病毒的中和抗体反应。LPR-repRNA-RBD 疫苗减少了小鼠的 SARS-CoV-2 感染和肺部炎症。总而言之,这些数据表明 LPR-repRNA 平台可以成为 COVID-19 疫苗开发的有前途的途径。





























 京公网安备 11010802027423号
京公网安备 11010802027423号