Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2023-04-23 , DOI: 10.1016/j.bmc.2023.117288 Dongyan Gu 1 , Mengmeng Zhang 2 , Lvtao Cai 1 , Chang Wang 2 , Yu-Bo Zhou 2 , Jia Li 2 , Rong Sheng 1
|
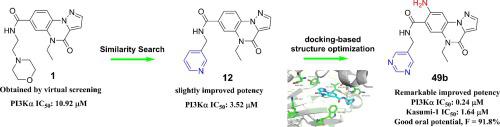
Compound 1 with pyrazolo[1,5-a]quinoxalin-4(5H)-one scaffold was identified as a PI3Kα inhibitor hit via virtual screening strategy. Additional similarity search and molecular docking based structural modification yielded a novel series of pyrazolo[1,5-a]quinoxalin-4(5H)-one derivatives. The most potent compound 49b exhibited remarkably improved PI3Kα inhibitory activity with IC50 value of 0.24 μM and moderate to good isoform selectivity over other class I PI3K isoforms. In addition, 49b significantly inhibited the proliferation of Kasumi-1 and T47D cells with IC50 value of 1.64 and 1.82 μM, respectively. Further PK study demonstrated that it has favorable pharmacokinetic profiles (AUC0-t = 3294.05 ng·h/mL at 5.0 mg/kg PO, F = 91.8%). All these data indicated that compound 49b was a promising PI3Kα inhibitor with beneficial drug-like properties and merited further development.
中文翻译:

通过虚拟筛选和基于对接的结构优化发现 4-oxo-4,5-dihydropyrazolo[1,5-a]quinoxaline-7-carboxamide 衍生物作为 PI3Kα 抑制剂
具有吡唑并 [1,5- a ]quinoxalin-4(5H)-one 支架的化合物 1 通过虚拟筛选策略被鉴定为 PI3Kα 抑制剂命中。额外的相似性搜索和基于分子对接的结构修饰产生了一系列新的 pyrazolo[1,5- a ]quinoxalin-4(5H)-one 衍生物。最有效的化合物49b表现出显着改善的 PI3Kα 抑制活性,IC 50值为 0.24 μM,并且与其他 I 类 PI3K 异构体相比具有中等至良好的异构体选择性。此外,49b显着抑制 Kasumi-1 和 T47D 细胞的增殖,IC 为50值分别为 1.64 和 1.82 μM。进一步的 PK 研究表明它具有良好的药代动力学特征(AUC 0-t = 3294.05 ng·h/mL at 5.0 mg/kg PO,F = 91.8%)。所有这些数据表明,化合物49b是一种很有前途的 PI3Kα 抑制剂,具有有益的药物样特性,值得进一步开发。

































 京公网安备 11010802027423号
京公网安备 11010802027423号