European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2023-04-23 , DOI: 10.1016/j.ejmech.2023.115402
Na Li 1 , Qi Guan 1 , Yilang Hong 1 , Bowen Zhang 1 , Mi Li 1 , Xuewen Li 2 , Bo Li 2 , Lan Wu 3 , Weige Zhang 1
|
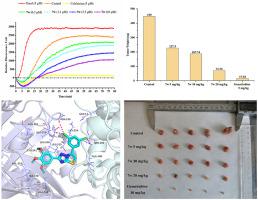
Tubulin/colchicine-binding site inhibitors (CBSIs) co-crystal structures play an important role in the exploration of novel small molecules for oncotherapy. Based on the analysis of the binding models of tubulin and reported CBSIs, a series of 6-aryl-2-(3,4,5-trimethoxyphenyl)thiazole[3,2-b][1,2,4]triazoles were designed as potential tubulin polymerization inhibitors by binding to distinct colchicine domain of tubulin. Among the compounds synthesized, 7w not only shown noteworthy potency against SGC-7901 cancer cell line (IC50 = 0.21 μM) but also exhibited lower cytotoxicity than colchicine in normal cell line (HUVEC). The mechanism studies elucidated that 7w could cause the apoptosis of cancer cells by inhibiting tubulin polymerization to trigger G2/M arrest. In 4T1-xenograft mice model, 7w significantly inhibited tumor growth without losing weight, demonstrating a promising potential for further development with a unique binding mode at the colchicine-binding site.
中文翻译:

发现 6-aryl-2-(3,4,5-trimethoxyphenyl)thiazole[3,2-b][1,2,4]triazoles 作为有效的微管蛋白聚合抑制剂
微管蛋白/秋水仙碱结合位点抑制剂 (CBSI) 共晶结构在探索用于肿瘤治疗的新型小分子中发挥着重要作用。基于对微管蛋白结合模型的分析和已报道的 CBSI,设计了一系列 6-aryl-2-(3,4,5-trimethoxyphenyl)thiazole[3,2- b ][1,2,4]triazoles通过与微管蛋白的不同秋水仙碱结构域结合,作为潜在的微管蛋白聚合抑制剂。在合成的化合物中,7w不仅对 SGC-7901 癌细胞系 (IC 50 = 0.21 μM) 表现出显着的效力,而且在正常细胞系 (HUVEC) 中表现出比秋水仙碱更低的细胞毒性。机制研究表明7w可通过抑制微管蛋白聚合引发 G 2 /M 阻滞,从而引起癌细胞凋亡。在 4T1 异种移植小鼠模型中,7w在不减轻体重的情况下显着抑制肿瘤生长,证明了在秋水仙碱结合位点具有独特结合模式的进一步开发的潜力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号