当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Double Oxidative [3 + 2] Cycloaddition for the Synthesis of CF3-Containing Spiro[pyrrolidin-3,2′-oxindole]
Organic Letters ( IF 4.9 ) Pub Date : 2023-04-21 , DOI: 10.1021/acs.orglett.3c01083 Tao Wang 1 , Wen-Bin Wang 1 , Yan-Ming Fu 1 , Cheng-Feng Zhu 1 , Lan-Jun Cheng 1 , Yang-En You 1 , Xiang Wu 1 , You-Gui Li 1
Organic Letters ( IF 4.9 ) Pub Date : 2023-04-21 , DOI: 10.1021/acs.orglett.3c01083 Tao Wang 1 , Wen-Bin Wang 1 , Yan-Ming Fu 1 , Cheng-Feng Zhu 1 , Lan-Jun Cheng 1 , Yang-En You 1 , Xiang Wu 1 , You-Gui Li 1
Affiliation
|
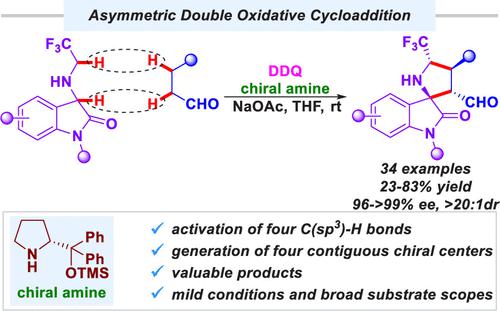
An asymmetric double oxidative [3 + 2] cycloaddition is reported. Oxidation of 3-((2,2,2-trifluoroethyl)amino)indolin-2-ones and β-aryl-substituted aldehydes simultaneously and subsequent asymmetric cycloaddition in the presence of the chiral amino catalyst generated highly functionalized chiral CF3-containing spiro[pyrrolidin-3,2′-oxindole] with four contiguous stereocenters stereoselectively, which is characterized by directly constructing two C–C bonds from four C(sp3)–H bonds. This new method features mild conditions, broad substrate scope, and excellent functional group compatibility.
中文翻译:

不对称双氧化 [3 + 2] 环加成法合成含 CF3 的螺[pyrrolidin-3,2'-oxindole]
报道了不对称双氧化 [3 + 2] 环加成。在手性氨基催化剂存在下,同时氧化 3-((2,2,2-trifluoroethyl)amino)indolin-2-ones 和 β-aryl-substitutional aldehydes 以及随后的不对称环加成生成高度官能化的手性 CF 3螺环[pyrrolidin-3,2'-oxindole] 具有四个连续的立构中心立体选择性,其特征在于由四个 C(sp 3 )–H 键直接构建两个 C–C 键。这种新方法具有条件温和、底物适用范围广、官能团相容性好等特点。
更新日期:2023-04-21
中文翻译:

不对称双氧化 [3 + 2] 环加成法合成含 CF3 的螺[pyrrolidin-3,2'-oxindole]
报道了不对称双氧化 [3 + 2] 环加成。在手性氨基催化剂存在下,同时氧化 3-((2,2,2-trifluoroethyl)amino)indolin-2-ones 和 β-aryl-substitutional aldehydes 以及随后的不对称环加成生成高度官能化的手性 CF 3螺环[pyrrolidin-3,2'-oxindole] 具有四个连续的立构中心立体选择性,其特征在于由四个 C(sp 3 )–H 键直接构建两个 C–C 键。这种新方法具有条件温和、底物适用范围广、官能团相容性好等特点。































 京公网安备 11010802027423号
京公网安备 11010802027423号