Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Less-Coordinated Atomic Copper-Dimer Boosted Carbon–Carbon Coupling During Electrochemical CO2 Reduction
Small ( IF 13.0 ) Pub Date : 2023-04-20 , DOI: 10.1002/smll.202301536 Kang Yang 1 , Yuntong Sun 1 , Sheng Chen 1 , Ming Li 1 , Min Zheng 2 , Lushan Ma 1 , Wenjun Fan 3 , Yao Zheng 2 , Qiang Li 1 , Jingjing Duan 1
Small ( IF 13.0 ) Pub Date : 2023-04-20 , DOI: 10.1002/smll.202301536 Kang Yang 1 , Yuntong Sun 1 , Sheng Chen 1 , Ming Li 1 , Min Zheng 2 , Lushan Ma 1 , Wenjun Fan 3 , Yao Zheng 2 , Qiang Li 1 , Jingjing Duan 1
Affiliation
|
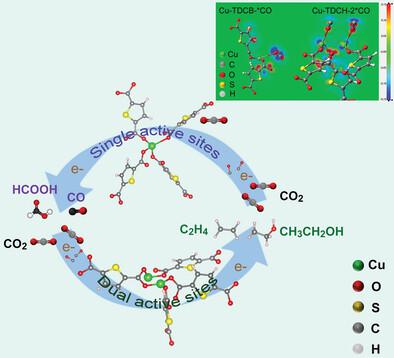
This work reports a metal–organic framework (MOF) with less-coordinated copper dimers, which displays excellent electrochemical CO2 reduction (eCO2RR) performance with an advantageous current density of 0.9 A cm−2 and a high Faradaic efficiency of 71% to C2 products. In comparison with MOF with Cu monomers that are present as Cu1O4 with a coordination number of 3.8 ± 0.2, Cu dimers exist as O3Cu1···Cu2O2 with a coordination number of 2.8 ± 0.1. In situ characterizations together with theoretical calculations reveal that two *CO intermediates are stably adsorbed on each site of less-coordinated Cu dimers, which favors later dimerization via a key intermediate of *CH2CHO. The highly unsaturated dual-atomic Cu provides large-quantity and high-quality actives sites for carbon–carbon coupling, achieving the optimal trade-off between activity and selectivity of eCO2RR to C2 products.
中文翻译:

电化学二氧化碳还原过程中较少配位的原子铜二聚体促进碳-碳偶联
这项工作报道了一种具有较少配位的铜二聚体的金属有机框架(MOF),它表现出优异的电化学CO 2还原(eCO 2 RR)性能,具有0.9 A cm -2的有利电流密度和71%的高法拉第效率至C 2产品。与Cu单体以配位数为3.8±0.2的Cu 1 O 4形式存在的MOF相比, Cu二聚体以配位数为2.8±0.1的O 3 Cu 1 ·Cu 2 O 2形式存在。原位表征和理论计算表明,两个 *CO 中间体稳定吸附在配位较少的 Cu 二聚体的每个位点上,这有利于随后通过 *CH 2 CHO关键中间体进行二聚化。高度不饱和的双原子Cu为碳-碳偶联提供了大量、高质量的活性位点,实现了eCO 2 RR到C 2产物的活性和选择性之间的最佳平衡。
更新日期:2023-04-20
中文翻译:

电化学二氧化碳还原过程中较少配位的原子铜二聚体促进碳-碳偶联
这项工作报道了一种具有较少配位的铜二聚体的金属有机框架(MOF),它表现出优异的电化学CO 2还原(eCO 2 RR)性能,具有0.9 A cm -2的有利电流密度和71%的高法拉第效率至C 2产品。与Cu单体以配位数为3.8±0.2的Cu 1 O 4形式存在的MOF相比, Cu二聚体以配位数为2.8±0.1的O 3 Cu 1 ·Cu 2 O 2形式存在。原位表征和理论计算表明,两个 *CO 中间体稳定吸附在配位较少的 Cu 二聚体的每个位点上,这有利于随后通过 *CH 2 CHO关键中间体进行二聚化。高度不饱和的双原子Cu为碳-碳偶联提供了大量、高质量的活性位点,实现了eCO 2 RR到C 2产物的活性和选择性之间的最佳平衡。































 京公网安备 11010802027423号
京公网安备 11010802027423号