当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Single-Atom Cu Channel and N-Vacancy Engineering Enables Efficient Charge Separation and Transfer between C3N4 Interlayers for Boosting Photocatalytic Hydrogen Production
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-04-21 , DOI: 10.1021/acscatal.2c05789
Jiachao Shen 1 , Chenghui Luo 1 , Shanshan Qiao 2 , Yuqing Chen 1 , Yanhong Tang 2 , Jieqiong Xu 1 , Kaixing Fu 1 , Dingwang Yuan 2 , Haifang Tang 1 , Hao Zhang 3 , Chengbin Liu 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-04-21 , DOI: 10.1021/acscatal.2c05789
Jiachao Shen 1 , Chenghui Luo 1 , Shanshan Qiao 2 , Yuqing Chen 1 , Yanhong Tang 2 , Jieqiong Xu 1 , Kaixing Fu 1 , Dingwang Yuan 2 , Haifang Tang 1 , Hao Zhang 3 , Chengbin Liu 1
Affiliation
|
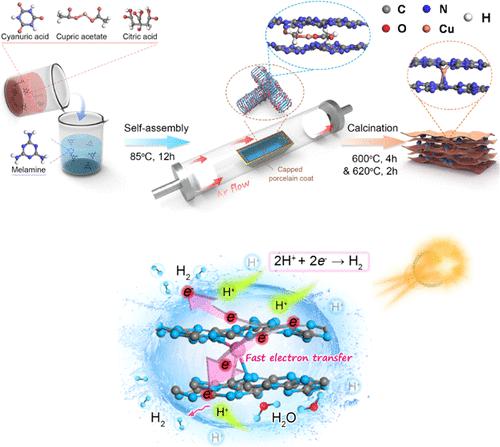
Polymeric carbon nitride (C3N4) has attracted great attention in photocatalysis due to its low-cost, visible-light response, and environment-friendly merits. However, the catalytic efficiency of pristine bulk C3N4 is severely limited by its poor photoinduced electron/hole pair separation and interlayer charge transport. Herein, single-atom Cu is bridged into C3N4 sheet interlayers through the thermal condensation of self-assembly supramolecules of Cu precursors and melamine–cyanuric acid monomers. Simultaneously, N vacancies are engineered into C3N4 only by gradient temperature. The single-atom Cu bridges serve as electron channels to promote photoinduced electron/hole pair separation and interlayer charge transport. The experimental results and calculations demonstrate that N vacancies break the symmetry of pristine C3N4, allowing more electrons to pass through the delocalized π-conjugated network of C3N4 to Cu sites, which facilitates charge transfer between C3N4 layers, resulting in more effective separation of electron/hole pairs, optimal charge distribution, and lower hydrogen evolution barrier. As a result, the photocatalyst at a stationary point with a 1 wt % Pt cocatalyst presents a high visible-light photocatalytic hydrogen production rate (11.23 mmol g–1 h–1), reaching a high apparent quantum yield of 31.60% at 420 nm. It is noted that the photocatalyst still exhibits a high hydrogen production rate of 605.15 μmol g–1 h–1 in the absence of the Pt cocatalyst.
中文翻译:

单原子 Cu 通道和 N 空位工程可实现 C3N4 中间层之间的有效电荷分离和转移,以促进光催化制氢
聚合物氮化碳(C 3 N 4)由于其低成本、可见光响应和环境友好等优点在光催化领域引起了极大的关注。然而,原始块状 C 3 N 4的催化效率受到其差的光致电子/空穴对分离和层间电荷传输的严重限制。在此,单原子 Cu通过 Cu 前体和三聚氰胺-氰尿酸单体的自组装超分子的热缩合桥接成 C 3 N 4片层夹层。同时,N个空位被设计成C 3 N 4仅通过梯度温度。单原子铜桥作为电子通道促进光致电子/空穴对分离和层间电荷传输。实验结果和计算表明,N空位打破了原始C 3 N 4的对称性,允许更多电子通过C 3 N 4的离域π-共轭网络到达Cu位点,这促进了C 3 N 4之间的电荷转移层,从而更有效地分离电子/空穴对,优化电荷分布,并降低析氢势垒。因此,该光催化剂在固定点与 1 wt% Pt 助催化剂呈现出高可见光光催化产氢率 (11.23 mmol g –1 h –1 ),在 420 nm 处达到 31.60% 的高表观量子产率. 值得注意的是,在没有 Pt 助催化剂的情况下,光催化剂仍然表现出 605.15 μmol g –1 h –1的高产氢率。
更新日期:2023-04-21
中文翻译:

单原子 Cu 通道和 N 空位工程可实现 C3N4 中间层之间的有效电荷分离和转移,以促进光催化制氢
聚合物氮化碳(C 3 N 4)由于其低成本、可见光响应和环境友好等优点在光催化领域引起了极大的关注。然而,原始块状 C 3 N 4的催化效率受到其差的光致电子/空穴对分离和层间电荷传输的严重限制。在此,单原子 Cu通过 Cu 前体和三聚氰胺-氰尿酸单体的自组装超分子的热缩合桥接成 C 3 N 4片层夹层。同时,N个空位被设计成C 3 N 4仅通过梯度温度。单原子铜桥作为电子通道促进光致电子/空穴对分离和层间电荷传输。实验结果和计算表明,N空位打破了原始C 3 N 4的对称性,允许更多电子通过C 3 N 4的离域π-共轭网络到达Cu位点,这促进了C 3 N 4之间的电荷转移层,从而更有效地分离电子/空穴对,优化电荷分布,并降低析氢势垒。因此,该光催化剂在固定点与 1 wt% Pt 助催化剂呈现出高可见光光催化产氢率 (11.23 mmol g –1 h –1 ),在 420 nm 处达到 31.60% 的高表观量子产率. 值得注意的是,在没有 Pt 助催化剂的情况下,光催化剂仍然表现出 605.15 μmol g –1 h –1的高产氢率。

































 京公网安备 11010802027423号
京公网安备 11010802027423号