当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility and Polymorphism of a HOF Monomer N1,N3,N5-Tris(pyridin-4-yl)benzene-1,3,5-tricarboxamide in 12 Neat Solvents at Temperatures from 283.15 to 323.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2023-04-21 , DOI: 10.1021/acs.jced.3c00039 Dandan Liu , Shen Hu , Yue Zhao , Yongjie Wang , Shujing Zhang , Jiaxin Wang , Jun Wang , Peng Wang , Zhenyu Li
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2023-04-21 , DOI: 10.1021/acs.jced.3c00039 Dandan Liu , Shen Hu , Yue Zhao , Yongjie Wang , Shujing Zhang , Jiaxin Wang , Jun Wang , Peng Wang , Zhenyu Li
|
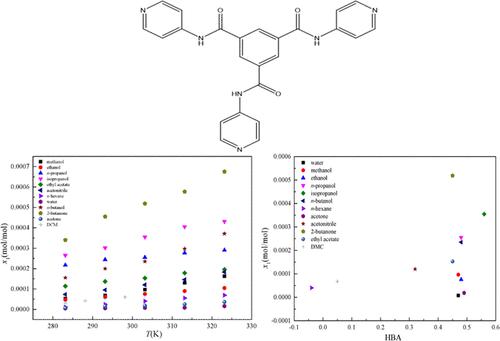
A gravimetric method is adopted to measure the solid–liquid equilibrium of N1,N3,N5-tris(pyridin-4-yl)benzene-1,3,5-tricarboxamide (TPBTC), a hydrogen-bonded organic framework (HOF) monomer in different solvents (methanol, dichloromethane, isopropanol, n-hexane, acetone, ethanol, 2-butanone, n-butanol, acetonitrile, water, ethyl acetate, and n-propanol). The experiment is carried out at atmospheric pressure, and the experimental temperatures range from 283.15 to 323.15 K. The powder X-ray diffraction analysis was performed on the equilibrium solid-phase characterization of TPBTC in different solvents, and the solid form of solute in equilibrium with different solvents was a mixture of three kinds of crystalline forms and an amorphous form. In the 12 monosolvents, the solubility increased with absolute temperature, and the following order was obtained: 2-butanone > isopropanol > n-propanol > n-butanol > ethyl acetate > acetonitrile > methanol > ethanol > dichloromethane > n-hexane > acetone > water. In the studied temperature range, the maximum and minimum values were recorded for 2-butanone (0.6753 × 10–3 mol/mol at 323.15 K) and water (0.004650 × 10–3 mol/mol at 283.15 K), respectively. The solubility behavior and solvent effects in various solvents were first investigated by the hydrogen bond acceptor tendencies as the main factor then interpreted by solvent polarity, cohesive energy density, and steric effect for some exceptions. In addition, the solid–liquid equilibrium data of TPBTC were correlated with the Yaws model and the Apelblat model. Furthermore, the two thermodynamic models were evaluated using the Akaike Information Criterion and Akaike weights. It can be observed that the Yaws model was more appropriate than the Apelblat model.
中文翻译:

HOF 单体 N1,N3,N5-Tris(pyridin-4-yl)benzene-1,3,5-tricarboxamide 在 283.15 至 323.15 K 温度下在 12 种纯溶剂中的溶解度和多态性
采用重量分析法测量N 1 , N 3 , N 5 -tris(pyridin-4-yl)benzene-1,3,5-tricarboxamide (TPBTC) 的固液平衡,TPBTC 是一种氢键有机骨架 ( HOF)单体在不同溶剂(甲醇、二氯甲烷、异丙醇、正己烷、丙酮、乙醇、2-丁酮、正丁醇、乙腈、水、乙酸乙酯和正-丙醇)。实验在常压下进行,实验温度范围为283.15~323.15 K。粉末X射线衍射分析了TPBTC在不同溶剂中的平衡固相表征,平衡态溶质的固体形态与不同的溶剂是三种结晶形式和无定形形式的混合物。在12种单一溶剂中,溶解度随绝对温度升高而增加,依次为:2-丁酮>异丙醇>正丙醇>正丁醇>乙酸乙酯>乙腈>甲醇>乙醇>二氯甲烷>正-己烷>丙酮>水。在研究的温度范围内,2-丁酮( 323.15 K 时为 0.6753 × 10 –3 mol/mol)和水(283.15 K 时为 0.004650 × 10 –3 mol/mol)分别记录了最大值和最小值。首先通过氢键受体倾向作为主要因素研究各种溶剂中的溶解度行为和溶剂效应,然后通过溶剂极性、内聚能密度和一些例外的空间效应来解释。此外,TPBTC的固液平衡数据与Yaws模型和Apelblat模型相关。此外,使用 Akaike 信息准则和 Akaike 权重评估了两个热力学模型。可以看出 Yaws 模型比 Apelblat 模型更合适。
更新日期:2023-04-21
中文翻译:

HOF 单体 N1,N3,N5-Tris(pyridin-4-yl)benzene-1,3,5-tricarboxamide 在 283.15 至 323.15 K 温度下在 12 种纯溶剂中的溶解度和多态性
采用重量分析法测量N 1 , N 3 , N 5 -tris(pyridin-4-yl)benzene-1,3,5-tricarboxamide (TPBTC) 的固液平衡,TPBTC 是一种氢键有机骨架 ( HOF)单体在不同溶剂(甲醇、二氯甲烷、异丙醇、正己烷、丙酮、乙醇、2-丁酮、正丁醇、乙腈、水、乙酸乙酯和正-丙醇)。实验在常压下进行,实验温度范围为283.15~323.15 K。粉末X射线衍射分析了TPBTC在不同溶剂中的平衡固相表征,平衡态溶质的固体形态与不同的溶剂是三种结晶形式和无定形形式的混合物。在12种单一溶剂中,溶解度随绝对温度升高而增加,依次为:2-丁酮>异丙醇>正丙醇>正丁醇>乙酸乙酯>乙腈>甲醇>乙醇>二氯甲烷>正-己烷>丙酮>水。在研究的温度范围内,2-丁酮( 323.15 K 时为 0.6753 × 10 –3 mol/mol)和水(283.15 K 时为 0.004650 × 10 –3 mol/mol)分别记录了最大值和最小值。首先通过氢键受体倾向作为主要因素研究各种溶剂中的溶解度行为和溶剂效应,然后通过溶剂极性、内聚能密度和一些例外的空间效应来解释。此外,TPBTC的固液平衡数据与Yaws模型和Apelblat模型相关。此外,使用 Akaike 信息准则和 Akaike 权重评估了两个热力学模型。可以看出 Yaws 模型比 Apelblat 模型更合适。






























 京公网安备 11010802027423号
京公网安备 11010802027423号