当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism-Driven Development of N-(Quinolin-8-yl)-benzamide Coupling Reactions via C–H or N–H Activation
Organometallics ( IF 2.5 ) Pub Date : 2023-04-20 , DOI: 10.1021/acs.organomet.2c00654 Egor L. Dolengovski 1 , Tatyana V. Gryaznova 1 , Yulia B. Dudkina 1 , Daut R. Islamov 2 , Robert R. Fayzullin 1 , Oleg G. Sinyashin 1 , Yulia H. Budnikova 1
Organometallics ( IF 2.5 ) Pub Date : 2023-04-20 , DOI: 10.1021/acs.organomet.2c00654 Egor L. Dolengovski 1 , Tatyana V. Gryaznova 1 , Yulia B. Dudkina 1 , Daut R. Islamov 2 , Robert R. Fayzullin 1 , Oleg G. Sinyashin 1 , Yulia H. Budnikova 1
Affiliation
|
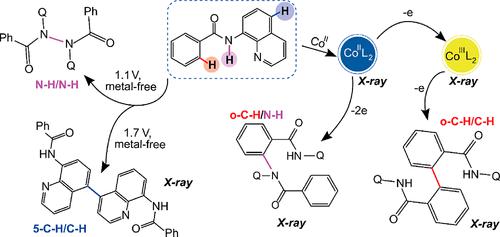
The mechanistic details of the oxidative coupling of compounds with a number of different redox centers are investigated using N-(quinolin-8-yl)-benzamide (L) as a model substrate. The control of the chemical or electrooxidation parameters in the absence or presence of a cobalt catalyst makes it possible to obtain regioselectively different oxidative coupling products (ortho- vs para-C–H/C–H vs C–H/N–H vs N–H/N–H). The results indicate that the operative mechanism depends on the type of oxidized reaction center and the oxidant nature. Oxidation affects the para-C–H bond in quinoline or the N–H fragment or the ortho-C–H bond in the benzene substituent in the molecule. The intermediate Co(II)[L–H]2 complex and C–H-activated CoIII metallacycle with benzamide ligands, which are shown to oxidize at close potentials, have been isolated and characterized by various techniques, including X-ray diffraction and voltammetry. The strength of the oxidizing agent affects the formation of a particular product, though not acting as the determining factor. Two-electron oxidation of Co(II)[L–H]2 yields to C–N coupling, but one-electron oxidation of Co(III) leads to ortho-C–C coupling. All electrochemical reactions are performed under mild conditions at room temperature without adding special reagents (oxidants, halides, phosphines, etc.).
中文翻译:

通过 C–H 或 N–H 激活的 N-(喹啉-8-基)-苯甲酰胺偶联反应的机制驱动发展
使用N- (喹啉-8-基)-苯甲酰胺 (L) 作为模型底物,研究了具有多个不同氧化还原中心的化合物氧化偶联的机制细节。在不存在或存在钴催化剂的情况下控制化学或电氧化参数,可以获得区域选择性不同的氧化偶联产物(邻位-对位-C–H/C–H vs C–H/N–H vs N –H/N–H)。结果表明,其作用机制取决于氧化反应中心的类型和氧化剂的性质。氧化影响喹啉中的对位-C-H键或分子中苯取代基中的N-H片段或邻位-C-H键。中间体Co(II)[L –H] 2配合物和带有苯甲酰胺配体的C-H 激活的 Co III金属环,显示出在接近电位下氧化,已通过各种技术(包括 X 射线衍射和伏安法)进行分离和表征。氧化剂的强度影响特定产物的形成,但不是决定因素。Co(II)[L –H ] 2的双电子氧化产生 C–N 偶联,但 Co(III) 的单电子氧化导致邻位-C–C 偶联。所有电化学反应均在室温温和条件下进行,无需添加特殊试剂(氧化剂、卤化物、膦等)。
更新日期:2023-04-20
中文翻译:

通过 C–H 或 N–H 激活的 N-(喹啉-8-基)-苯甲酰胺偶联反应的机制驱动发展
使用N- (喹啉-8-基)-苯甲酰胺 (L) 作为模型底物,研究了具有多个不同氧化还原中心的化合物氧化偶联的机制细节。在不存在或存在钴催化剂的情况下控制化学或电氧化参数,可以获得区域选择性不同的氧化偶联产物(邻位-对位-C–H/C–H vs C–H/N–H vs N –H/N–H)。结果表明,其作用机制取决于氧化反应中心的类型和氧化剂的性质。氧化影响喹啉中的对位-C-H键或分子中苯取代基中的N-H片段或邻位-C-H键。中间体Co(II)[L –H] 2配合物和带有苯甲酰胺配体的C-H 激活的 Co III金属环,显示出在接近电位下氧化,已通过各种技术(包括 X 射线衍射和伏安法)进行分离和表征。氧化剂的强度影响特定产物的形成,但不是决定因素。Co(II)[L –H ] 2的双电子氧化产生 C–N 偶联,但 Co(III) 的单电子氧化导致邻位-C–C 偶联。所有电化学反应均在室温温和条件下进行,无需添加特殊试剂(氧化剂、卤化物、膦等)。

































 京公网安备 11010802027423号
京公网安备 11010802027423号