Synthesis ( IF 2.2 ) Pub Date : 2023-04-19 , DOI: 10.1055/a-2055-7678 Chanyoung Boo 1 , Hyein Kim 1 , Huijeong Yang 1 , Seunghyo Han 1 , Huisu Yeo 1 , Chibeom Seo 1 , Sangho Koo 2
|
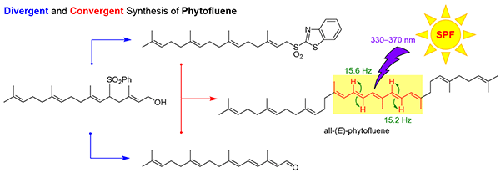
Practical synthetic methods for biogenetically and pharmaceutically important phytofluene were developed through the divergent preparation of key C20 substrates from a common intermediate and convergent synthesis by Wittig and Julia–Kocienski olefinations. Expeditious synthesis of phytofluene was also proposed based on the Julia sulfone-mediated chain-extension and double elimination method. Stereochemical outcomes of these olefination methods for phytofluene were compared and the Julia–Kocienski method was the mildest and most efficient reaction condition to produce all-(E)-phytofluene. Complete 1H and 13C NMR analysis of all-(E)-phytofluene is reported for the first time. Phytofluene undergoes facile thermal isomerization to other Z-isomers above room temperature, which was also confirmed with a C30 phytofluene homologue.
中文翻译:

发散中间体的制备及植物氟的趋同合成
生物遗传学和药学上重要的植物氟烯的实用合成方法是通过从普通中间体中分离制备关键的 C 20底物以及通过 Wittig 和 Julia–Kocienski 烯烃化聚合合成而开发的。还提出了基于 Julia 砜介导的扩链和双消除法快速合成植物氟烯。比较了这些植物氟烯化方法的立体化学结果,Julia–Kocienski 方法是生产全 ( E )-植物氟烯的最温和、最有效的反应条件。完成所有-( E的1 H 和13 C NMR 分析)-phytofluene 为首次报道。植物氟在室温以上容易热异构化为其他Z异构体,这也用 C 30植物氟同系物证实。































 京公网安备 11010802027423号
京公网安备 11010802027423号