Cell Reports Physical Science ( IF 7.9 ) Pub Date : 2023-04-19 , DOI: 10.1016/j.xcrp.2023.101379 Xinyu Ma , Jiangtao Yu , Xiuyang Zou , Yin Hu , Mingchen Yang , Feng Zhang , Feng Yan
|
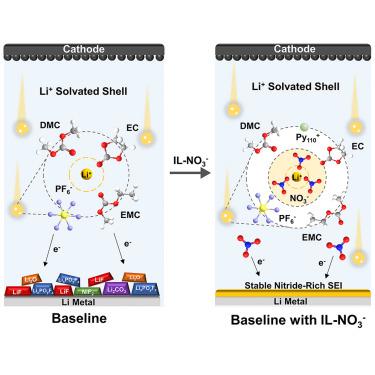
Lithium nitrate (LiNO3) is widely used to build a stable and highly Li+ conductive solid electrode/electrolyte interface (SEI) for ether-based electrolytes. However, the low solubility of LiNO3 in carbonate-based electrolytes limits its applications in high-voltage lithium-metal batteries. Herein, we report a strategy to introduce NO3− into carbonate electrolytes by a single ionic liquid-type additive, 1-decyl-1-methylpyrrolidine (Py110+) nitrate (IL–NO3–), without additional solubilizers to manipulate Li+ solvation structure. Compared with LiNO3, the weak binding energy between Py110+ and NO3− in IL–NO3– enables carbonate solvent molecules to easily break the IL–NO3– cluster. The NO3− participating in the Li+ solvation sheath can form a robust and highly conductive SEI to promote Li+ transport kinetics. This work provides a realistic reference for the application of an ionic liquid additive to build a stable SEI for advancing the development of lithium-metal batteries.
中文翻译:

用于调节高性能锂金属电池碳酸盐电解质中锂离子溶剂化结构的单一添加剂
硝酸锂 (LiNO 3 ) 广泛用于为醚基电解质构建稳定且高 Li +导电性的固体电极/电解质界面 (SEI)。然而,LiNO 3在碳酸盐基电解质中的低溶解度限制了其在高压锂金属电池中的应用。在此,我们报告了一种通过单一离子液体型添加剂 1-decyl-1-methylpyrrolidine (Py 110 + ) 硝酸盐 (IL–NO 3 – )将 NO 3 −引入碳酸盐电解质的策略,无需额外的增溶剂来操纵 Li +溶剂化结构。与LiNO 3相比,Py之间的结合能弱IL–NO 3 中的110 +和NO 3 −使碳酸盐溶剂分子能够轻松破坏 IL–NO 3 –簇。参与 Li +溶剂化鞘层的NO 3 -可以形成坚固且高导电的 SEI,以促进 Li +传输动力学。该工作为应用离子液体添加剂构建稳定的SEI以推进锂金属电池的发展提供了现实参考。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号