当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanisms of pH-Dependent Activity for Water Oxidation to Molecular Oxygen by MnO2Electrocatalysts
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2012-01-13 , DOI: 10.1021/ja206511w Toshihiro Takashima 1 , Kazuhito Hashimoto 1, 2 , Ryuhei Nakamura 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2012-01-13 , DOI: 10.1021/ja206511w Toshihiro Takashima 1 , Kazuhito Hashimoto 1, 2 , Ryuhei Nakamura 1
Affiliation
|
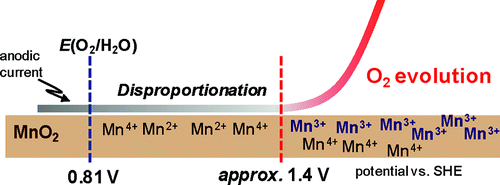
Manganese oxides function as efficient electrocatalysts for water oxidation to molecular oxygen in strongly alkaline conditions, but are inefficient at neutral pH. To provide new insight into the mechanism underlying the pH-dependent activity of the electrooxidation reaction, we performed UV-vis spectroelectrochemical detection of the intermediate species for water oxidation by a manganese oxide electrode. Layered manganese oxide nanoparticles, δ-MnO(2) (K(0.17)[Mn(4+)(0.90)Mn(3+)(0.07)□(0.03)]O(2)·0.53H(2)O) deposited on fluorine-doped tin oxide electrodes were shown to catalyze water oxidation at pH from 4 to 13. At this pH range, a sharp rise in absorption at 510 nm was observed with a concomitant increase of anodic current for O(2) evolution. Using pyrophosphate as a probe molecule, the 510 nm absorption was attributable to Mn(3+) on the surface of δ-MnO(2). The onset potential of the water oxidation current was constant at approximately 1.5 V vs SHE from pH 4 to pH 8, but sharply shifted to negative at pH > 8. Strikingly, this behavior was well reproduced by the pH dependence of the onset of 510 nm absorption, indicating that Mn(3+) acts as the precursor of water oxidation. Mn(3+) is unstable at pH < 9 due to the disproportionation reaction resulting in the formation of Mn(2+) and Mn(4+), whereas it is effectively stabilized by the comproportionation of Mn(2+) and Mn(4+) in alkaline conditions. Thus, the low activity of manganese oxides for water oxidation under neutral conditions is most likely due to the inherent instability of Mn(3+), whose accumulation at the surface of catalysts requires the electrochemical oxidation of Mn(2+) at a potential of approximately 1.4 V. This new model suggests that the control of the disproportionation and comproportionation efficiencies of Mn(3+) is essential for the development of Mn catalysts that afford water oxidation with a small overpotential at neutral pH.
中文翻译:

MnO2 电催化剂将水氧化为分子氧的 pH 依赖性活性机制
锰氧化物在强碱性条件下可作为将水氧化为分子氧的有效电催化剂,但在中性 pH 值下效率低下。为了提供对电氧化反应的 pH 依赖性活性的潜在机制的新见解,我们通过氧化锰电极对水氧化的中间物质进行了紫外-可见光谱电化学检测。层状氧化锰纳米粒子,δ-MnO(2) (K(0.17)[Mn(4+)(0.90)Mn(3+)(0.07)□(0.03)]O(2)·0.53H(2)O)沉积在掺氟氧化锡电极上的 4 到 13 的 ph 值催化水氧化。在这个 ph 值范围内,观察到 510 nm 处的吸收急剧上升,同时增加了 O(2) 演化的阳极电流。使用焦磷酸盐作为探针分子,510 nm 的吸收归因于 δ-MnO(2) 表面上的 Mn(3+)。从 pH 4 到 pH 8,水氧化电流的起始电位相对于 SHE 恒定在大约 1.5 V,但在 pH > 8 时急剧变为负值。 引人注目的是,这种行为通过 510 nm 起始的 pH 依赖性很好地重现吸收,表明 Mn(3+) 作为水氧化的前体。由于歧化反应导致形成 Mn(2+) 和 Mn(4+),Mn(3+) 在 pH < 9 时不稳定,而通过 Mn(2+) 和 Mn( 4+) 在碱性条件下。因此,锰氧化物在中性条件下对水氧化的低活性很可能是由于 Mn(3+) 的固有不稳定性,
更新日期:2012-01-13
中文翻译:

MnO2 电催化剂将水氧化为分子氧的 pH 依赖性活性机制
锰氧化物在强碱性条件下可作为将水氧化为分子氧的有效电催化剂,但在中性 pH 值下效率低下。为了提供对电氧化反应的 pH 依赖性活性的潜在机制的新见解,我们通过氧化锰电极对水氧化的中间物质进行了紫外-可见光谱电化学检测。层状氧化锰纳米粒子,δ-MnO(2) (K(0.17)[Mn(4+)(0.90)Mn(3+)(0.07)□(0.03)]O(2)·0.53H(2)O)沉积在掺氟氧化锡电极上的 4 到 13 的 ph 值催化水氧化。在这个 ph 值范围内,观察到 510 nm 处的吸收急剧上升,同时增加了 O(2) 演化的阳极电流。使用焦磷酸盐作为探针分子,510 nm 的吸收归因于 δ-MnO(2) 表面上的 Mn(3+)。从 pH 4 到 pH 8,水氧化电流的起始电位相对于 SHE 恒定在大约 1.5 V,但在 pH > 8 时急剧变为负值。 引人注目的是,这种行为通过 510 nm 起始的 pH 依赖性很好地重现吸收,表明 Mn(3+) 作为水氧化的前体。由于歧化反应导致形成 Mn(2+) 和 Mn(4+),Mn(3+) 在 pH < 9 时不稳定,而通过 Mn(2+) 和 Mn( 4+) 在碱性条件下。因此,锰氧化物在中性条件下对水氧化的低活性很可能是由于 Mn(3+) 的固有不稳定性,




















































 京公网安备 11010802027423号
京公网安备 11010802027423号