当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Site- and Stereoselective Synthesis of Alkenyl Chlorides by Dual Functionalization of Internal Alkynes via Photoredox/Nickel Catalysis
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-04-18 , DOI: 10.1021/jacs.3c02748 Liping Huo 1 , Xiaofang Li 1 , Yaheng Zhao 1 , Ling Li 1 , Lingling Chu 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-04-18 , DOI: 10.1021/jacs.3c02748 Liping Huo 1 , Xiaofang Li 1 , Yaheng Zhao 1 , Ling Li 1 , Lingling Chu 1
Affiliation
|
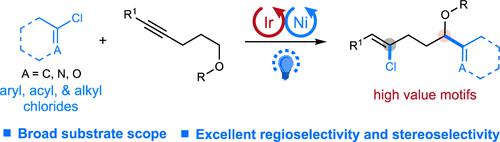
Herein, we report a redox-neutral and atom-economical protocol to synthesize valuable alkenyl chlorides from unactivated internal alkynes and abundant organochlorides via photoredox and nickel catalysis. This protocol enables the site- and stereoselective addition of organochlorides to alkynes via chlorine photoelimination-initiated sequential hydrochlorination/remote C–H functionalization. The protocol is compatible with a wide range of medicinally relevant heteroaryl, aryl, acid, and alkyl chlorides for efficiently producing γ-functionalized alkenyl chlorides, exhibiting excellent regioselectivities and stereoselectivities. Late-stage modifications and synthetic manipulations of the products and preliminary mechanistic studies are also presented.
中文翻译:

光氧化还原/镍催化内部炔烃双功能化定点和立体选择性合成烯基氯
在此,我们报告了一种氧化还原中性和原子经济方案,通过光氧化还原和镍催化从未活化的内部炔烃和丰富的有机氯化物中合成有价值的烯基氯。该协议能够通过氯光消除引发的顺序氢氯化/远程 C-H 功能化,将有机氯化物定点和立体选择性地加成到炔烃中。该协议与广泛的医学相关的杂芳基、芳基、酸和烷基氯兼容,可有效生产 γ 功能化的烯基氯,表现出出色的区域选择性和立体选择性。还介绍了产品的后期修改和合成操作以及初步的机理研究。
更新日期:2023-04-18
中文翻译:

光氧化还原/镍催化内部炔烃双功能化定点和立体选择性合成烯基氯
在此,我们报告了一种氧化还原中性和原子经济方案,通过光氧化还原和镍催化从未活化的内部炔烃和丰富的有机氯化物中合成有价值的烯基氯。该协议能够通过氯光消除引发的顺序氢氯化/远程 C-H 功能化,将有机氯化物定点和立体选择性地加成到炔烃中。该协议与广泛的医学相关的杂芳基、芳基、酸和烷基氯兼容,可有效生产 γ 功能化的烯基氯,表现出出色的区域选择性和立体选择性。还介绍了产品的后期修改和合成操作以及初步的机理研究。






























 京公网安备 11010802027423号
京公网安备 11010802027423号