Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2023-04-18 , DOI: 10.1016/j.molstruc.2023.135608 Zeid Owidha , Rayan M. Alansari , Moustafa A. Gouda , Belal H.M. Hussein
|
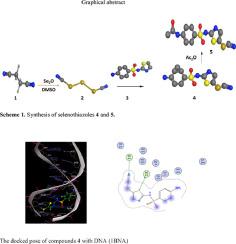
Triselenium dicyanide (TSD, 2) was combined with 4-amino-N-(thiazol-2-yl) benzenesulfonamide (3) in DMSO to form 4-amino-N-(5-selenocyanatothiazol-2-yl) benzenesulfonamide (4). Acetamide 5 was formed by acetylation of seleno derivative 4 with acetic anhydride. The absorption and fluorescence methods were used to monitor the interaction of compounds 3–5 with the double strain fish sperm deoxyribonucleic acid (dsFS-DNA), revealing a groove binding interaction with a binding constant in the order of 104 M−1. The measured Gibbs free energies, entropies, and enthalpy changes indicated that all interaction processes were spontaneous, with the hydrogen bonding and van der Waals interactions playing important participation for the binding of 3-5 with FS-DNA. Based on the molecular modeling results, the compound binds to the groove of B-DNA with a relative binding energy of -5.06 to -5.94 kcal mol−1. Biological investigations revealed that compound 4 was the most cytotoxic against Panc1 cells with IC50 value of 9.3 µg/mL compared to Doxorubicin (IC50=3.16 µg/mL). It induced apoptosis in Panc1 cells, increasing the death rate by 31.38-fold; it induced total apoptosis by 20.4% (8.2% for early apoptosis, 22.2 % for late apoptosis) compared to 0.65% in the untreated control group. Additionally, compound 4 and 5 exhibited remarkable antioxidant activity with a DPPH inhibition ratio of 71.6 and 56.6% compared to Trolox (96%) at the highest concentration of 100 μM. The findings of this research offer additional useful insights regarding organoselenium compound-DNA interactions as promising cytotoxic agents through apoptosis.
中文翻译:

一种新型 4-氨基-N-(5-硒代氰基噻唑-2-基) 苯磺酰胺的合成、DNA 结合、分子对接和细胞毒活性
三硒二氰化物 (TSD, 2) 与 4-氨基-N- (噻唑-2-基) 苯磺酰胺 (3) 在 DMSO 中结合形成 4-氨基-N- (5-硒代氰基噻唑-2-基) 苯磺酰胺 (4) . 乙酰胺 5 是由硒代衍生物 4 用乙酸酐乙酰化形成的。吸收和荧光方法用于监测化合物 3-5 与双品系鱼精脱氧核糖核酸 (dsFS-DNA) 的相互作用,揭示了结合常数为 10 4 M −1 数量级的凹槽结合相互作用. 测量的吉布斯自由能、熵和焓变表明所有相互作用过程都是自发的,氢键和范德瓦尔斯相互作用对 3-5 与FS -DNA 的结合起着重要的作用。根据分子建模结果,该化合物以-5.06 至-5.94 kcal mol -1的相对结合能结合B-DNA 的沟槽。生物学研究表明化合物 4 对 Panc1 细胞的细胞毒性最强,IC 50值为 9.3 µg/ mL=3.16 微克/毫升)。诱导Panc1细胞凋亡,死亡率增加31.38倍;它诱导了 20.4% 的总细胞凋亡(早期细胞凋亡为 8.2%,晚期细胞凋亡为 22.2%),而未处理的对照组为 0.65%。此外,与最高浓度 100 μM 的 Trolox (96%) 相比,化合物 4 和 5 表现出显着的抗氧化活性,DPPH 抑制率为 71.6% 和 56.6%。这项研究的结果提供了关于有机硒化合物-DNA 相互作用作为有前途的细胞毒性药物通过细胞凋亡的额外有用见解。








































 京公网安备 11010802027423号
京公网安备 11010802027423号