Tetrahedron Letters ( IF 1.5 ) Pub Date : 2023-04-18 , DOI: 10.1016/j.tetlet.2023.154509
Jian Zhang , Wen-Sheng Li , Sen Lu , Wen-Juan Wan , Li-Xin Wang
|
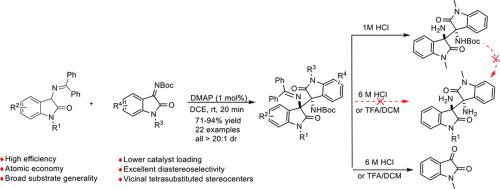
A highly diastereoselective (all > 20:1 dr) and effective (1 mol% cat) new Mannich reaction between 3‑amino oxindole Schiff base and oxindole ketimines promoted by catalytic DMAP under mild conditions has been disclosed. Highly steric 3,3′-bisoxindole protected diamines with adjacent tetrasubstituted stereocenters were conveniently and directly obtained in high yields and excellent diastereoselectivities (all > 20:1 dr) within a few minutes. A further preparation of the adjacent tetrasubstituted 3,3′-bisoxindole free diamines by general deprotection lead to the collapse of the diamine scaffold. A typical scale-up preparation, partial hydrolysis of the product and subsequent transformations of the resulting free monoamine with phenylcyanate and phenylisothiocyanate was smoothly performed to form new bisoxindole ureas and thioureas. Our protocol and the easily obtained new diversity of bisoxindoles may find potential pharmaceutical and catalytic applications.
中文翻译:

3-氨基羟吲哚席夫碱与羟吲哚酮亚胺之间的高度非对映选择性曼尼希反应——直接有效制备相邻四取代 3,3'-双羟吲哚保护的二胺
已经公开了在温和条件下由催化 DMAP 促进的3-氨基羟吲哚席夫碱和羟吲哚酮亚胺之间的高度非对映选择性(所有 > 20:1 dr)和有效(1 mol% cat)新曼尼希反应。具有相邻四取代立构中心的高度立体 3,3'-双氧吲哚保护的二胺可在几分钟内对映选择性方便、直接地获得通过一般脱保护进一步制备相邻的四取代 3,3'-双氧吲哚游离二胺会导致二胺支架崩溃。典型的放大制备、产品的部分水解和所得游离单胺与苯氰酸酯和异硫氰酸苯酯的反应顺利进行,形成了新的双氧吲哚脲和硫脲。我们的协议和容易获得的新多样性 bisoxindoles 可能会发现潜在的制药和催化应用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号