Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2023-04-18 , DOI: 10.1016/j.cej.2023.143001
Mengchen Hu , Tianqi Yu , Kexin Tan , Anchao Zhou , Lin Luo , Shibin Yin
|
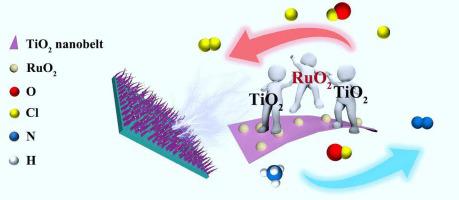
Chlorine evolution reaction (CER) usually requires catalyst with high content of noble metals for applications at large current density, thus hindering its industrial development. In this work, TiO2 nanobelts supported RuO2 nanoparticles (RuO2-TiO2 NBs-Ti) with ultralow Ru loading (0.83 wt%) is synthesized. It exhibits high activity for CER with 50 mA cm−2 at only 1.13 V (vs. SCE) and mass activity of 57.1 A g−1Ru at 1.14 V, surpassing the dimensionally stable anode (DSA). And the activity can stably maintain at large current density of 300 mA cm−2 for 408 h. Furthermore, it also shows high performance for degradation of NH4+−N (98.9% within 90 min). The ideal performance is ascribed to the strong oxide-support interaction (SOSI) that tunes the interfacial electronic structure between mixing antase-rutile-TiO2 and RuO2, which improves the Volmer-Heyrovsky kinetic of CER. Meanwhile, it possesses nanobelt structure with a larger specific surface area, which can provide more active sites and is beneficial to the escape of bubbles on the electrode surface. This work provides a prospective direction for the synthesis of low-cost and high-activity CER catalyst at large current density.
中文翻译:

超低 Ru 载量 RuO2-TiO2 具有强氧化物-载体相互作用,可实现有效的氯析出和氨氮消除
析氯反应(CER)在大电流密度下的应用通常需要贵金属含量高的催化剂,从而阻碍了其工业化发展。在这项工作中,合成了具有超低 Ru 负载量 (0.83 wt%) 的TiO 2纳米带负载 RuO 2纳米粒子 (RuO 2 -TiO 2 NBs-Ti)。它对 CER 表现出高活性,仅在 1.13 V(相对于SCE)时为 50 mA cm -2,在 1.14 V 时质量活性为 57.1 A g -1 Ru,超过尺寸稳定阳极 (DSA)。且活性在300 mA cm -2的大电流密度下仍能稳定保持408 小时。此外,它还显示出高效的 NH 4 + −N降解性能(90 分钟内 98.9%)。理想的性能归因于强氧化物-载体相互作用 (SOSI),它调节了混合的反钛矿-金红石-TiO 2和 RuO 2之间的界面电子结构,从而提高了 CER 的 Volmer-Heyrovsky 动力学。同时,它具有比表面积更大的纳米带结构,可以提供更多的活性位点,有利于电极表面气泡的逸出。该工作为在大电流密度下合成低成本、高活性的CER催化剂提供了一个有前景的方向。

































 京公网安备 11010802027423号
京公网安备 11010802027423号