当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of Spiroketal Alkaloids Lycibarbarines A–C
Organic Letters ( IF 4.9 ) Pub Date : 2023-04-17 , DOI: 10.1021/acs.orglett.3c00902
Eilidh G Young 1 , Phillip S Grant 1 , Daniel P Furkert 1, 2 , Margaret A Brimble 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2023-04-17 , DOI: 10.1021/acs.orglett.3c00902
Eilidh G Young 1 , Phillip S Grant 1 , Daniel P Furkert 1, 2 , Margaret A Brimble 1, 2
Affiliation
|
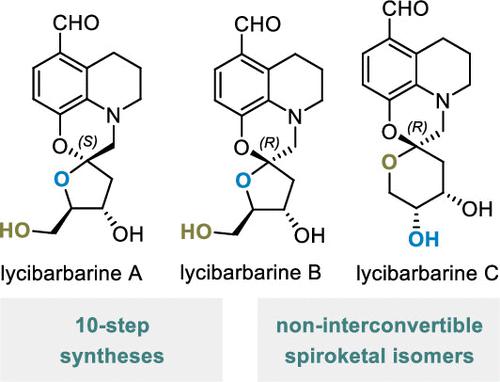
Lycibarbarines A–C are spirocyclic alkaloids with a unique tetracyclic framework, consisting of tetrahydroquinoline and spiro-fused oxazine–sugar spiroketal subunits. The first total syntheses of lycibarbarines A–C were achieved over 10 steps (longest linear sequence) each. Through this work, it was discovered that the spiroketal unit of lycibarbarines A–C exhibits unusually high resistance to acid-mediated isomerization and epimerization, likely due to the basic nitrogen atom. As such, the lycibarbarines present an interesting case study in preventing the interconversion of spiroketal isomers, which may prove to be instructive in efforts to obtain nonthermodynamic spiroketal frameworks.
中文翻译:

螺缩酮生物碱 Lycibarbarines A–C 的全合成
Lycibarbarines A–C 是具有独特四环框架的螺环生物碱,由四氢喹啉和螺稠恶嗪-糖螺缩酮亚基组成。lycibarbarines A–C 的第一次全合成是通过 10 个步骤(最长的线性序列)实现的。通过这项工作,人们发现 lycibarbarines A–C 的螺缩酮单元对酸介导的异构化和差向异构化表现出异常高的抵抗力,这可能是由于碱性氮原子。因此,lycibarbarines 在防止螺缩酮异构体相互转化方面提供了一个有趣的案例研究,这可能对获得非热力学螺缩酮框架的努力具有指导意义。
更新日期:2023-04-17
中文翻译:

螺缩酮生物碱 Lycibarbarines A–C 的全合成
Lycibarbarines A–C 是具有独特四环框架的螺环生物碱,由四氢喹啉和螺稠恶嗪-糖螺缩酮亚基组成。lycibarbarines A–C 的第一次全合成是通过 10 个步骤(最长的线性序列)实现的。通过这项工作,人们发现 lycibarbarines A–C 的螺缩酮单元对酸介导的异构化和差向异构化表现出异常高的抵抗力,这可能是由于碱性氮原子。因此,lycibarbarines 在防止螺缩酮异构体相互转化方面提供了一个有趣的案例研究,这可能对获得非热力学螺缩酮框架的努力具有指导意义。

































 京公网安备 11010802027423号
京公网安备 11010802027423号