当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ion Mobility-Mass Spectrometry and Collision-Induced Unfolding of Designed Bispecific Antibody Therapeutics
Analytical Chemistry ( IF 6.7 ) Pub Date : 2023-04-17 , DOI: 10.1021/acs.analchem.3c00344 Rosendo C Villafuerte-Vega 1 , Henry W Li 1 , Thomas R Slaney 2 , Naresh Chennamsetty 2 , Guodong Chen 2 , Li Tao 2 , Brandon T Ruotolo 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2023-04-17 , DOI: 10.1021/acs.analchem.3c00344 Rosendo C Villafuerte-Vega 1 , Henry W Li 1 , Thomas R Slaney 2 , Naresh Chennamsetty 2 , Guodong Chen 2 , Li Tao 2 , Brandon T Ruotolo 1
Affiliation
|
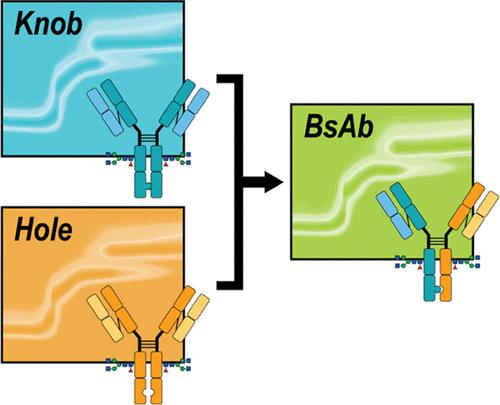
Bispecific antibodies (bsAbs) represent a critically important class of emerging therapeutics capable of targeting two different antigens simultaneously. As such, bsAbs have been developed as effective treatment agents for diseases that remain challenging for conventional monoclonal antibody (mAb) therapeutics to access. Despite these advantages, bsAbs are intricate molecules, requiring both the appropriate engineering and pairing of heavy and light chains derived from separate parent mAbs. Current analytical tools for tracking the bsAb construction process have demonstrated a limited ability to robustly probe the higher-order structure (HOS) of bsAbs. Native ion mobility-mass spectrometry (IM-MS) and collision-induced unfolding (CIU) have proven to be useful tools in probing the HOS of mAb therapeutics. In this report, we describe a series of detailed and quantitative IM-MS and CIU data sets that reveal HOS details associated with a knob-into-hole (KiH) bsAb model system and its corresponding parent mAbs. We find that quantitative analysis of CIU data indicates that global KiH bsAb stability occupies an intermediate space between the stabilities recorded for its parent mAbs. Furthermore, our CIU data identify the hole-containing half of the KiH bsAb construct to be the least stable, thus driving much of the overall stability of the KiH bsAb. An analysis of both intact bsAb and enzymatic fragments allows us to associate the first and second CIU transitions observed for the intact KiH bsAb to the unfolding Fab and Fc domains, respectively. This result is likely general for CIU data collected for low charge state mAb ions and is supported by data acquired for deglycosylated KiH bsAb and mAb constructs, each of which indicates greater destabilization of the second CIU transition observed in our data. When integrated, our CIU analysis allows us to link changes in the first CIU transition primarily to the Fab region of the hole-containing halfmer, while the second CIU transition is likely strongly connected to the Fc region of the knob-containing halfmer. Taken together, our results provide an unprecedented road map for evaluating the domain-level stabilities and HOS of both KiH bsAb and mAb constructs using CIU.
中文翻译:

设计的双特异性抗体疗法的离子淌度-质谱法和碰撞诱导展开
双特异性抗体 (bsAb) 代表了一类极其重要的新兴疗法,能够同时靶向两种不同的抗原。因此,bsAb 已被开发为有效的治疗药物,用于治疗传统单克隆抗体 (mAb) 疗法仍然具有挑战性的疾病。尽管有这些优点,bsAb 是复杂的分子,需要对源自单独亲本 mAb 的重链和轻链进行适当的工程设计和配对。目前用于跟踪 bsAb 构建过程的分析工具已证明稳健探测 bsAb 高阶结构 (HOS) 的能力有限。天然离子淌度质谱 (IM-MS) 和碰撞诱导解折叠 (CIU) 已被证明是探测 mAb 疗法 HOS 的有用工具。在本报告中,我们描述了一系列详细的定量 IM-MS 和 CIU 数据集,这些数据集揭示了与旋钮入孔 (KiH) bsAb 模型系统及其相应的亲本单克隆抗体相关的 HOS 详细信息。我们发现 CIU 数据的定量分析表明,KiH bsAb 的整体稳定性处于其亲本 mAb 记录的稳定性之间的中间位置。此外,我们的 CIU 数据确定 KiH bsAb 构建体中含有孔的一半是最不稳定的,从而在很大程度上推动了 KiH bsAb 的整体稳定性。对完整 bsAb 和酶片段的分析使我们能够将完整 KiH bsAb 观察到的第一个和第二个 CIU 转变分别与展开的 Fab 和 Fc 结构域相关联。对于低电荷态 mAb 离子收集的 CIU 数据来说,这一结果可能是普遍的,并且得到了去糖基化 KiH bsAb 和 mAb 构建体采集的数据的支持,其中每一个都表明我们的数据中观察到的第二个 CIU 转变的稳定性更大。整合后,我们的 CIU 分析使我们能够将第一个 CIU 转换的变化主要与含空穴半聚体的 Fab 区域联系起来,而第二个 CIU 转换可能与含旋钮半聚体的 Fc 区域密切相关。总而言之,我们的结果为使用 CIU 评估 KiH bsAb 和 mAb 构建体的域水平稳定性和 HOS 提供了前所未有的路线图。
更新日期:2023-04-17
中文翻译:

设计的双特异性抗体疗法的离子淌度-质谱法和碰撞诱导展开
双特异性抗体 (bsAb) 代表了一类极其重要的新兴疗法,能够同时靶向两种不同的抗原。因此,bsAb 已被开发为有效的治疗药物,用于治疗传统单克隆抗体 (mAb) 疗法仍然具有挑战性的疾病。尽管有这些优点,bsAb 是复杂的分子,需要对源自单独亲本 mAb 的重链和轻链进行适当的工程设计和配对。目前用于跟踪 bsAb 构建过程的分析工具已证明稳健探测 bsAb 高阶结构 (HOS) 的能力有限。天然离子淌度质谱 (IM-MS) 和碰撞诱导解折叠 (CIU) 已被证明是探测 mAb 疗法 HOS 的有用工具。在本报告中,我们描述了一系列详细的定量 IM-MS 和 CIU 数据集,这些数据集揭示了与旋钮入孔 (KiH) bsAb 模型系统及其相应的亲本单克隆抗体相关的 HOS 详细信息。我们发现 CIU 数据的定量分析表明,KiH bsAb 的整体稳定性处于其亲本 mAb 记录的稳定性之间的中间位置。此外,我们的 CIU 数据确定 KiH bsAb 构建体中含有孔的一半是最不稳定的,从而在很大程度上推动了 KiH bsAb 的整体稳定性。对完整 bsAb 和酶片段的分析使我们能够将完整 KiH bsAb 观察到的第一个和第二个 CIU 转变分别与展开的 Fab 和 Fc 结构域相关联。对于低电荷态 mAb 离子收集的 CIU 数据来说,这一结果可能是普遍的,并且得到了去糖基化 KiH bsAb 和 mAb 构建体采集的数据的支持,其中每一个都表明我们的数据中观察到的第二个 CIU 转变的稳定性更大。整合后,我们的 CIU 分析使我们能够将第一个 CIU 转换的变化主要与含空穴半聚体的 Fab 区域联系起来,而第二个 CIU 转换可能与含旋钮半聚体的 Fc 区域密切相关。总而言之,我们的结果为使用 CIU 评估 KiH bsAb 和 mAb 构建体的域水平稳定性和 HOS 提供了前所未有的路线图。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号