Nano Research ( IF 9.5 ) Pub Date : 2023-04-14 , DOI: 10.1007/s12274-023-5585-2 Qian Xu , Xingwang Cheng , Ningqiang Zhang , Yi Tu , Lihui Wu , Haibin Pan , Jun Hu , Honghe Ding , Junfa Zhu , Yadong Li
|
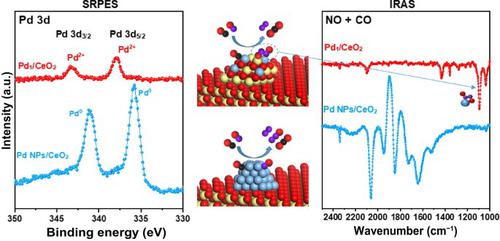
Selective catalytic reduction of NO by CO is challenging in environmental catalysis but attractive owing to the advantage of simultaneous elimination of NO and CO. Here, model catalysts consisting of Pd nanoparticles (NPs) and single-atom Pd supported on a CeO2 (111) film grown on Cu (111) (denoted as Pd NPs/CeO2 and Pd1/CeO2, respectively) were successfully prepared and characterized by synchrotron radiation photoemission spectroscopy (SRPES) and infrared reflection absorption spectroscopy (IRAS). The NO + CO adsorption/reaction on the Pd1/CeO2 and Pd NPs/CeO2 catalysts were carefully investigated using SRPES, temperature-programmed desorption (TPD), and IRAS. It is found that the reaction products on both model catalysts are in good agreement with those on real catalysts, demonstrating the good reliability of using these model catalysts to study the reaction mechanism of the NO + CO reaction. On the Pd NPs/CeO2 surface, N2 is formed by the combination of atomic N coming from the dissociation of NO on Pd NPs at higher temperatures. N2O formation occurs probably via chemisorbed NO combined with atomic N on the surface. While on the single-atom Pd1/CeO2 surface, no N2O is detected. The 100% N2 selectivity may stem from the formation of O-N-N-O* intermediate on the surface. Through this study, direct experimental evidence for the reaction mechanisms of the NO + CO reaction is provided, which supports the previous density functional theory (DFT) calculations.
中文翻译:

通过模型催化剂揭示 Pd/CeO2 单原子催化剂在 NO + CO 反应中的优势
通过 CO 选择性催化还原 NO 在环境催化中具有挑战性,但由于同时消除 NO 和 CO 的优势而具有吸引力。这里,模型催化剂由负载在 CeO 2 (111)上的 Pd 纳米粒子 (NPs) 和单原子 Pd 组成成功地制备了在 Cu (111) 上生长的薄膜(分别表示为 Pd NPs/CeO 2和 Pd 1 /CeO 2),并通过同步辐射光电子能谱(SRPES)和红外反射吸收光谱(IRAS)对其进行了表征。NO + CO在Pd 1 /CeO 2和Pd NPs/CeO 2上的吸附/反应使用 SRPES、程序升温脱附 (TPD) 和 IRAS 仔细研究了催化剂。发现两种模型催化剂上的反应产物与真实催化剂上的反应产物非常吻合,证明使用这些模型催化剂研究 NO + CO 反应的反应机理具有良好的可靠性。在 Pd NPs/CeO 2表面,N 2由来自 Pd NPs 上 NO 在较高温度下解离的原子 N 的结合形成。N 2 O 的形成可能是通过化学吸附的 NO 与表面上的原子 N 结合而发生的。而在单原子Pd 1 /CeO 2表面,没有检测到N 2 O。100% N 2选择性可能源于表面上 ONNO* 中间体的形成。通过这项研究,为NO + CO反应的反应机理提供了直接的实验证据,支持了之前的密度泛函理论(DFT)计算。































 京公网安备 11010802027423号
京公网安备 11010802027423号