Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2023-04-15 , DOI: 10.1016/j.molliq.2023.121841
Mohamed Shaker S. Adam
|
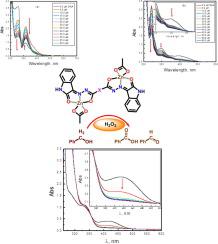
Two new dinuclear complexes of Zn2+ ion (ZnLC2 and ZnLC6, respectively) with two novel diisatin dihydrazone ligands of succinyl (H2LC2) and terephthalyl (H2LC2) derivatives were constructed. Their structure confirmation was achieved by different spectroscopic tools. The catalytic action of two ZnLCX was estimated under an aerobic atmosphere in the selective oxygenation protocol of benzyl alcohol within hydrogen peroxide. ZnLC6 represented more significant action by 88 % awarding of the monooxo-product, i.e. benzaldehyde, (with loaded catalyst amount = 0.005 mmol, at reaction temperature 70 °C for about 3 h), whereas ZnLC2 showed less reactivity with 87 % of the monooxo-product (with the same supplied amount, at 70 °C for 5 h).
The biochemical action of H2LCX and ZnLCX was studied spectroscopically with some microbial-series and three human cancer cells. In addition, their biological action was studied through the interacting mode with calf thymus DNA (ctDNA) through viscometric and spectrophotometric assays. Both ZnLCX complexing reagents manifested a regarded inhibiting action versus the titled microbial (bacterial and fungal) series and with three human cancer cells growth (according to the zone values in mm of inhibition, MIC in μM, and IC50 values in µM) than that of free H2LCX ligands. ZnLC6 exhibited a distinguished interacting ability with ctDNA more than that interaction behavior of ZnLC2 based on the derived magnitudes of Kb (the binding constants), and (the Gibbs’ free energy), respectively. Such results referred to the lipophilic feature of the terphthalyl derivative compare to that of the succinyl one of the bonded ligands. Also, both ZnLC2 and ZnLC6 exhibited an observable higher reactivity over their both H2LC2 and H2LC6 ligands pointing to the function of Zn2+ ion and the structural effect of the free ligands for their progressing reactivity against the studied bacterial series, fungal series, human cancer cell lines and ctDNA.
中文翻译:

Zn(II)-diisatin dihydrazones 的配合物作为苯甲醇氧化方案中的有效催化剂和用于生物学研究的有效试剂
构建了两个新的Zn 2+离子双核配合物(分别为ZnLC2 和ZnLC6)与琥珀酰基(H 2 LC2)和对苯二甲酰基(H 2 LC2)衍生物的两个新二靛红二腙配体。它们的结构确认是通过不同的光谱工具实现的。在苯甲醇在过氧化氢中的选择性氧化方案中,在有氧气氛下估计了两种 ZnLCX 的催化作用。ZnLC6 通过 88% 的单氧代产物(即苯甲醛)表现出更显着的作用(负载催化剂量 = 0.005 mmol,在反应温度 70 °C 下持续约 3 小时),而 ZnLC2 显示出与 87% 的单氧代产物的反应性较低-产品(与供应量相同,在 70°C 下 5 小时)。
用一些微生物系列和三种人类癌细胞对H 2 LCX 和 ZnLCX的生化作用进行了光谱研究。此外,通过粘度计和分光光度法测定,通过与小牛胸腺 DNA ( ct DNA) 的相互作用模式研究了它们的生物学作用。与标题微生物(细菌和真菌)系列相比,两种 ZnLCX 络合试剂均表现出明显的抑制作用,并且三种人类癌细胞生长(根据以毫米为单位的抑制区域值、以 μM 为单位的 MIC 和以 μM 为单位的 IC 50值)游离 H 2 LCX 配体。ZnLC6 表现出与ct显着的相互作用能力DNA 比 ZnLC2 的相互作用行为更多,基于K b的衍生大小(结合常数),和(吉布斯自由能)。此类结果涉及对苯二甲酰基衍生物与键合配体中的琥珀酰基衍生物相比的亲脂性特征。此外,ZnLC2 和 ZnLC6 都表现出比它们的 H 2 LC2 和 H 2 LC6 配体更高的反应性,这表明 Zn 2+离子的功能和游离配体的结构效应对于它们对所研究的细菌系列、真菌、真菌的进展反应性系列、人类癌细胞系和ct DNA。































 京公网安备 11010802027423号
京公网安备 11010802027423号