当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Monitoring and Inhibition of Insulin Fibrillation by a Small Organic Fluorogen with Aggregation-Induced Emission Characteristics
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2012-01-10 , DOI: 10.1021/ja208720a Yuning Hong 1 , Luming Meng 1 , Sijie Chen 1 , Chris Wai Tung Leung 1 , Lin-Tai Da 1 , Mahtab Faisal 1 , Daniel-Adriano Silva 1 , Jianzhao Liu 1 , Jacky Wing Yip Lam 1 , Xuhui Huang 1 , Ben Zhong Tang 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2012-01-10 , DOI: 10.1021/ja208720a Yuning Hong 1 , Luming Meng 1 , Sijie Chen 1 , Chris Wai Tung Leung 1 , Lin-Tai Da 1 , Mahtab Faisal 1 , Daniel-Adriano Silva 1 , Jianzhao Liu 1 , Jacky Wing Yip Lam 1 , Xuhui Huang 1 , Ben Zhong Tang 1
Affiliation
|
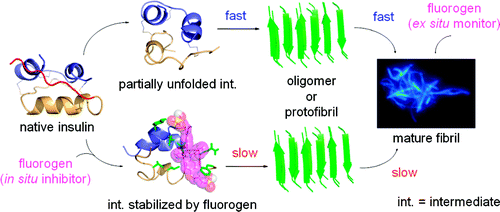
Amyloid fibrillation of proteins is associated with a great variety of pathologic conditions. Development of new molecules that can monitor amyloidosis kinetics and inhibit fibril formation is of great diagnostic and therapeutic value. In this work, we have developed a biocompatible molecule that functions as an ex situ monitor and an in situ inhibitor for protein fibrillation, using insulin as a model protein. 1,2-Bis[4-(3-sulfonatopropoxyl)phenyl]-1,2-diphenylethene salt (BSPOTPE) is nonemissive when it is dissolved with native insulin in an incubation buffer but starts to fluoresce when it is mixed with preformed insulin fibril, enabling ex situ monitoring of amyloidogenesis kinetics and high-contrast fluorescence imaging of protein fibrils. Premixing BSPOTPE with insulin, on the other hand, inhibits the nucleation process and impedes the protofibril formation. Increasing the dose of BSPOTPE boosts its inhibitory potency. Theoretical modeling using molecular dynamics simulations and docking reveals that BSPOTPE is prone to binding to partially unfolded insulin through hydrophobic interaction of the phenyl rings of BSPOTPE with the exposed hydrophobic residues of insulin. Such binding is assumed to have stabilized the partially unfolded insulin and obstructed the formation of the critical oligomeric species in the protein fibrillogenesis process.
中文翻译:

具有聚集诱导发射特性的小型有机荧光剂监测和抑制胰岛素纤颤
蛋白质的淀粉样蛋白纤维化与多种病理状况有关。开发可以监测淀粉样变性动力学和抑制原纤维形成的新分子具有重要的诊断和治疗价值。在这项工作中,我们开发了一种生物相容性分子,它使用胰岛素作为模型蛋白质,可用作异位监测器和蛋白质纤维化的原位抑制剂。1,2-双[4-(3-磺基丙氧基)苯基]-1,2-二苯基乙烯盐 (BSPOTPE) 在孵育缓冲液中与天然胰岛素一起溶解时不发光,但在与预制胰岛素原纤维混合时开始发出荧光,能够对淀粉样蛋白生成动力学进行非原位监测和蛋白质原纤维的高对比度荧光成像。另一方面,将 BSPOTPE 与胰岛素预混合,抑制成核过程并阻碍原纤维的形成。增加 BSPOTPE 的剂量可提高其抑制效力。使用分子动力学模拟和对接的理论建模表明 BSPOTPE 易于通过 BSPOTPE 的苯环与暴露的胰岛素疏水残基的疏水相互作用与部分未折叠的胰岛素结合。假设这种结合稳定了部分未折叠的胰岛素,并阻碍了蛋白质原纤维形成过程中关键寡聚物质的形成。使用分子动力学模拟和对接的理论建模表明 BSPOTPE 易于通过 BSPOTPE 的苯环与暴露的胰岛素疏水残基的疏水相互作用与部分未折叠的胰岛素结合。假设这种结合稳定了部分未折叠的胰岛素,并阻碍了蛋白质原纤维形成过程中关键寡聚物质的形成。使用分子动力学模拟和对接的理论建模表明 BSPOTPE 易于通过 BSPOTPE 的苯环与暴露的胰岛素疏水残基的疏水相互作用与部分未折叠的胰岛素结合。假设这种结合稳定了部分未折叠的胰岛素,并阻碍了蛋白质原纤维形成过程中关键寡聚物质的形成。
更新日期:2012-01-10
中文翻译:

具有聚集诱导发射特性的小型有机荧光剂监测和抑制胰岛素纤颤
蛋白质的淀粉样蛋白纤维化与多种病理状况有关。开发可以监测淀粉样变性动力学和抑制原纤维形成的新分子具有重要的诊断和治疗价值。在这项工作中,我们开发了一种生物相容性分子,它使用胰岛素作为模型蛋白质,可用作异位监测器和蛋白质纤维化的原位抑制剂。1,2-双[4-(3-磺基丙氧基)苯基]-1,2-二苯基乙烯盐 (BSPOTPE) 在孵育缓冲液中与天然胰岛素一起溶解时不发光,但在与预制胰岛素原纤维混合时开始发出荧光,能够对淀粉样蛋白生成动力学进行非原位监测和蛋白质原纤维的高对比度荧光成像。另一方面,将 BSPOTPE 与胰岛素预混合,抑制成核过程并阻碍原纤维的形成。增加 BSPOTPE 的剂量可提高其抑制效力。使用分子动力学模拟和对接的理论建模表明 BSPOTPE 易于通过 BSPOTPE 的苯环与暴露的胰岛素疏水残基的疏水相互作用与部分未折叠的胰岛素结合。假设这种结合稳定了部分未折叠的胰岛素,并阻碍了蛋白质原纤维形成过程中关键寡聚物质的形成。使用分子动力学模拟和对接的理论建模表明 BSPOTPE 易于通过 BSPOTPE 的苯环与暴露的胰岛素疏水残基的疏水相互作用与部分未折叠的胰岛素结合。假设这种结合稳定了部分未折叠的胰岛素,并阻碍了蛋白质原纤维形成过程中关键寡聚物质的形成。使用分子动力学模拟和对接的理论建模表明 BSPOTPE 易于通过 BSPOTPE 的苯环与暴露的胰岛素疏水残基的疏水相互作用与部分未折叠的胰岛素结合。假设这种结合稳定了部分未折叠的胰岛素,并阻碍了蛋白质原纤维形成过程中关键寡聚物质的形成。































 京公网安备 11010802027423号
京公网安备 11010802027423号