Chemical Engineering Science ( IF 4.1 ) Pub Date : 2023-04-14 , DOI: 10.1016/j.ces.2023.118751 Miyi Li , Yu Hu , Liqiang Lv , Tao Fang , Long Hao , Shenhui Li , Yufeng Wu , Xiao Dong , Helei Liu
|
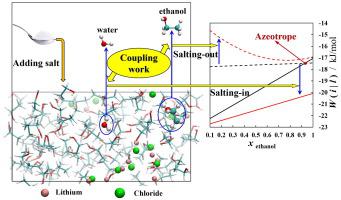
An azeotrope is a special vapor–liquid equilibrium in which the composition of two coexisting phases is equal. Due to its strong deviation from the Raoult’s law, it poses a great challenge to the simple distillation process and the understanding of fundamental molecular mechanisms. In this study, we calculated the coupling work of solute species based on the solvation free energy framework to predict the vapor–liquid equilibrium (VLE) behavior and azeotrope point of mixtures. Two prototypical binary mixtures: the negative water-hydrazine and positive ethanol–water azeotrope were investigated using molecular dynamic simulations and the OPLS-AA force field. All the simulations were performed with the TIP3P water model, because it has a good capability to represent enthalpy properties. The molecular models of hydrazine and ethanol have been re-parameterized to reproduce the density and solvation free energy properties of pure substance. Using free energy perturbation simulation, the coupling work of each substance was studied against liquid mixture composition. The coupling work is also translated to x-y or T-x-y phase diagrams for comparison with available experimental data. The tendency of two azeotropes is that the more volatile components experience the less pronounced coupling work changes in their local environments as the azeotropes form. The coupling work is also divided into van der Waals and Coulomb contributions to investigate the interactions between particles. Lithium Chloride was investigated as an entrainer for the separation of ethanol–water system. The coupling works of binary mixtures were calculated at different salt concentrations to study the salt-out effect for the violate species and salt-in effect for the less violate species. By adding LiCl, it was found that the azeotrope point was efficiently eliminated at a salinity 0.05 mol fraction at 1 bar. All the results show that the simulation methodology of coupling work are reliable for calculating the liquid–vapor equilibrium behavior. This method is also valuable for selecting and designing new extractants for distillation process.
中文翻译:

实施溶剂化自由能框架来预测水-肼和乙醇-水共沸系统的汽液平衡行为
共沸物是一种特殊的汽液平衡,其中两个共存相的组成相等。由于其强烈偏离拉乌尔定律,对简单的蒸馏过程和对基本分子机制的理解提出了巨大挑战。在这项研究中,我们基于溶剂化自由能框架计算了溶质物质的耦合功,以预测混合物的气液平衡 (VLE) 行为和共沸点。使用分子动力学模拟和 OPLS-AA 力场研究了两种典型的二元混合物:负水-肼和正乙醇-水共沸物。所有模拟都是使用 TIP3P 水模型进行的,因为它具有很好的表示焓特性的能力。对肼和乙醇的分子模型进行了重新参数化,以重现纯物质的密度和溶剂化自由能特性。使用自由能微扰模拟,针对液体混合物成分研究了每种物质的耦合功。耦合工作也被翻译成x - y或T - x - y用于与现有实验数据进行比较的相图。两种共沸物的趋势是,当共沸物形成时,挥发性更强的组分在其局部环境中经历不太明显的耦合功变化。耦合工作也分为范德瓦尔斯和库仑贡献,以研究粒子之间的相互作用。研究了氯化锂作为乙醇-水系统分离的夹带剂。计算二元混合物在不同盐浓度下的耦合功,以研究违反物种的盐析效应和较少违反物种的盐入效应。通过添加 LiCl,发现共沸点在 1 bar 下盐度为 0.05 摩尔分数时有效消除。所有结果表明,耦合工作的模拟方法对于计算液汽平衡行为是可靠的。该方法对于选择和设计新的精馏萃取剂也有一定的参考价值。






























 京公网安备 11010802027423号
京公网安备 11010802027423号