背景与目标
非酒精性脂肪性肝炎(NASH)驱动的肝细胞癌(HCC)的患病率正在迅速上升,但其潜在机制仍不清楚。在此,我们的目的是确定缺氧诱导脂滴相关蛋白(HILPDA)/缺氧诱导基因 2 (HIG2)(细胞内脂肪分解的选择性抑制剂)在 NASH 驱动的 HCC 中的作用。
方法
通过免疫组织化学和转录组学分析评估了人类 NASH 驱动的 HCC 标本中 HILPDA 的临床意义。在暴露于游离脂肪酸和常氧或缺氧的情况下,在人类 HCC 细胞和 3D 上皮球体中评估了 HILPDA 的致癌作用。通过鸟枪法和靶向方法评估野生型和HILPDA敲除 HCC 细胞的脂质组学分析。野生型 ( Hilpda fl/fl ) 和肝细胞特异性Hilpda敲除 ( Hilpda ΔHep ) 小鼠被喂食西方饮食和高糖饮用水,同时接受四氯化碳以诱导 NASH 驱动的 HCC。
结果
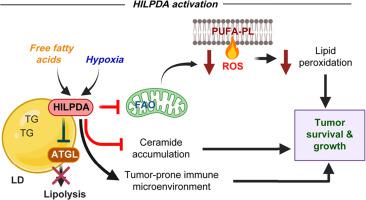
在 NASH 驱动的 HCC 患者中,HILPDA 表达上调与较差的生存率密切相关。在缺氧和高脂培养条件下,HILPDA 可促进人肝癌细胞的活力和 3D 上皮球体的生长。 HILPDA 的缺乏会引发多不饱和脂肪酸向膜磷脂的流动以及饱和脂肪酸向神经酰胺合成的流动,从而加剧缺氧时的脂质过氧化和细胞凋亡。 HILPDA 缺乏诱导的细胞凋亡可通过神经酰胺合成的药理抑制来逆转。在我们的 NASH 驱动的 HCC 实验小鼠模型中, Hilpda ΔHep表现出肝脏脂肪变性和肿瘤发生减少,但肝脏氧化应激增加。单细胞分析支持肝脏 HILPDA 在保护 HCC 细胞和促进 NASH 中促肿瘤免疫微环境的建立方面发挥双重作用。
结论
肝脏 HILPDA 是 NASH 肝脏微环境中的关键肿瘤代谢因子,代表了潜在的新型治疗靶点。
影响和影响
非酒精性脂肪性肝炎(NASH,由脂肪堆积、肝脏炎症和损伤引起的慢性代谢性肝病)正在成为肝细胞癌 (HCC)(最常见的肝癌形式)的主要危险因素和增长最快的原因。虽然 HCC 存在治愈性治疗选择,但它经常出现在晚期,此时这些选择不再有效,只能进行全身治疗。然而,全身治疗仍然存在疗效不佳和一些副作用的问题。此外,尚无批准用于 NASH 的药物。因此,了解 NASH 驱动的 HCC 期间发生的潜在代谢变化是确定针对癌细胞独特代谢需求的新癌症治疗方法的关键。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
HILPDA promotes NASH-driven HCC development by restraining intracellular fatty acid flux in hypoxia
Background & Aims
The prevalence of non-alcoholic steatohepatitis (NASH)-driven hepatocellular carcinoma (HCC) is rising rapidly, yet its underlying mechanisms remain unclear. Herein, we aim to determine the role of hypoxia-inducible lipid droplet associated protein (HILPDA)/hypoxia-inducible gene 2 (HIG2), a selective inhibitor of intracellular lipolysis, in NASH-driven HCC.
Methods
The clinical significance of HILPDA was assessed in human NASH-driven HCC specimens by immunohistochemistry and transcriptomics analyses. The oncogenic effect of HILPDA was assessed in human HCC cells and in 3D epithelial spheroids upon exposure to free fatty acids and either normoxia or hypoxia. Lipidomics profiling of wild-type and HILPDA knockout HCC cells was assessed via shotgun and targeted approaches. Wild-type (Hilpdafl/fl) and hepatocyte-specific Hilpda knockout (HilpdaΔHep) mice were fed a Western diet and high sugar in drinking water while receiving carbon tetrachloride to induce NASH-driven HCC.
Results
In patients with NASH-driven HCC, upregulated HILPDA expression is strongly associated with poor survival. In oxygen-deprived and lipid-loaded culture conditions, HILPDA promotes viability of human hepatoma cells and growth of 3D epithelial spheroids. Lack of HILPDA triggered flux of polyunsaturated fatty acids to membrane phospholipids and of saturated fatty acids to ceramide synthesis, exacerbating lipid peroxidation and apoptosis in hypoxia. The apoptosis induced by HILPDA deficiency was reversed by pharmacological inhibition of ceramide synthesis. In our experimental mouse model of NASH-driven HCC, HilpdaΔHep exhibited reduced hepatic steatosis and tumorigenesis but increased oxidative stress in the liver. Single-cell analysis supports a dual role of hepatic HILPDA in protecting HCC cells and facilitating the establishment of a pro-tumorigenic immune microenvironment in NASH.
Conclusions
Hepatic HILPDA is a pivotal oncometabolic factor in the NASH liver microenvironment and represents a potential novel therapeutic target.
Impact and implications
Non-alcoholic steatohepatitis (NASH, chronic metabolic liver disease caused by buildup of fat, inflammation and damage in the liver) is emerging as the leading risk factor and the fastest growing cause of hepatocellular carcinoma (HCC), the most common form of liver cancer. While curative therapeutic options exist for HCC, it frequently presents at a late stage when such options are no longer effective and only systemic therapies are available. However, systemic therapies are still associated with poor efficacy and some side effects. In addition, no approved drugs are available for NASH. Therefore, understanding the underlying metabolic alterations occurring during NASH-driven HCC is key to identifying new cancer treatments that target the unique metabolic needs of cancer cells.






















































 京公网安备 11010802027423号
京公网安备 11010802027423号