European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2023-04-14 , DOI: 10.1016/j.ejmech.2023.115381
Ziqin Yan 1 , Xilin Lyu 1 , Dongze Lin 2 , Gaoxing Wu 3 , Yang Gong 4 , Xuelian Ren 5 , Jian Xiao 6 , Jianfeng Lou 3 , He Huang 7 , Yi Chen 2 , Yujun Zhao 8
|
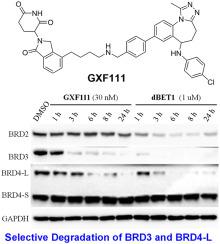
Targeted degradation of BET family proteins BRD2/3/4 or only BRD4 with PROTAC molecules has been a promising strategy for the treatment of human cancer. Meanwhile, selective degradation of cellular BRD3 and BRD4-L remains a challenging task. We report herein a novel PROTAC molecule 24 that promoted selective degradation of cellular BRD3 and BRD4-L, but not BRD2 or BRD4-S, in a panel of six cancer cell lines. The observed target selectivity was partially attributed to differences in protein degradation kinetics and in types of cell lines. In a MM.1S mouse xenograft model, an optimized lead compound 28 promoted selective degradation of BRD3 and BRD4-L in vivo and exhibited robust antitumor activity. In summary, we have demonstrated that selective degradation of BRD3 and BRD4-L over BRD2 and BRD4-S is a feasible and robust approach in multiple cancer cell lines and an animal model, which could be helpful for further investigations on BRD3 and BRD4-L that ultimately benefitting cancer research and therapeutics.
中文翻译:

PROTAC 分子在六种癌细胞系中促进细胞 BRD3 和 BRD4-L 的选择性降解
使用 PROTAC 分子靶向降解 BET 家族蛋白 BRD2/3/4 或仅降解 BRD4 一直是治疗人类癌症的有前途的策略。同时,细胞 BRD3 和 BRD4-L 的选择性降解仍然是一项具有挑战性的任务。我们在此报告了一种新型 PROTAC 分子24 ,它促进了一组六种癌细胞系中细胞 BRD3 和 BRD4-L 而不是 BRD2 或 BRD4-S 的选择性降解。观察到的目标选择性部分归因于蛋白质降解动力学和细胞系类型的差异。在 MM.1S 小鼠异种移植模型中,优化的先导化合物28促进了 BRD3 和 BRD4-L在体内的选择性降解并表现出强大的抗肿瘤活性。总之,我们已经证明,在多种癌细胞系和动物模型中,BRD3 和 BRD4-L 相对于 BRD2 和 BRD4-S 的选择性降解是一种可行且稳健的方法,这可能有助于进一步研究 BRD3 和 BRD4-L最终有利于癌症研究和治疗。

































 京公网安备 11010802027423号
京公网安备 11010802027423号