当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Anodic Solid Electrolyte Interphase Formed around the Li2OHBr/LiCoO2 Interface
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2023-04-14 , DOI: 10.1021/acsaem.2c04103
Keisuke Yoshikawa 1 , Yasuhiro Suzuki 1, 2 , Akihiro Shiota 2 , Yasutoshi Iriyama 1
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2023-04-14 , DOI: 10.1021/acsaem.2c04103
Keisuke Yoshikawa 1 , Yasuhiro Suzuki 1, 2 , Akihiro Shiota 2 , Yasutoshi Iriyama 1
Affiliation
|
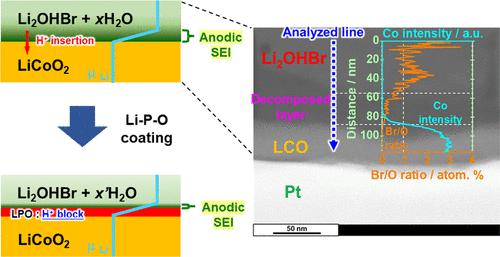
4 V-class oxide-based all-solid-state batteries, Li/Li2OHBr/LiCoO2, are prepared using ductile antiperovskite-structured Li2OHBr via room-temperature densification. The effects of anodic decomposition of Li2OHBr on the LiCoO2/Li2OHBr interfacial resistance and charge–discharge stabilities are investigated. Li2OHBr decomposes on LiCoO2 at 3.6 V vs. Li/Li+, and an irreversible charging capacity is observed in the first cycle. This anodic decomposition does not increase the resistance at the LiCoO2/Li2OHBr interface and in Li2OHBr. Transmission electron microscopy analysis shows an anodic-decomposed region of Li2OHBr with a thickness of ∼30 nm between the LiCoO2 and Li2OHBr phases. This thickness is almost consistent with the expected value assuming a one- to two-electron anodic decomposition reaction of Li2OHBr. The resulting solid electrolyte interphase will have acceptable Li+ conductivity and improves the adhesion of the interface. The surface coating of amorphous lithium phosphate stabilizes charge–discharge reactions probably by suppressing proton insertion into LiCoO2.
中文翻译:

Li2OHBr/LiCoO2 界面周围形成的阳极固体电解质界面
4 V 级氧化物基全固态电池 Li/Li 2 OHBr/LiCoO 2是使用延展性反钙钛矿结构的 Li 2 OHBr 通过室温致密化制备的。研究了 Li 2 OHBr 的阳极分解对 LiCoO 2 /Li 2 OHBr 界面电阻和充放电稳定性的影响。Li 2 OHBr 在 LiCoO 2上在 3.6 V vs. Li/Li +下分解,并且在第一个循环中观察到不可逆的充电容量。这种阳极分解不会增加 LiCoO 2 /Li 2 OHBr 界面和 Li 2中的电阻OHBr。透射电子显微镜分析显示 Li 2 OHBr的阳极分解区域在 LiCoO 2和 Li 2 OHBr 相之间具有约 30 nm 的厚度。该厚度几乎与假设 Li 2 OHBr的单电子到双电子阳极分解反应的预期值一致。所得固体电解质界面将具有可接受的 Li +电导率并改善界面的粘附性。无定形磷酸锂的表面涂层可能通过抑制质子插入 LiCoO 2来稳定充放电反应。
更新日期:2023-04-14
中文翻译:

Li2OHBr/LiCoO2 界面周围形成的阳极固体电解质界面
4 V 级氧化物基全固态电池 Li/Li 2 OHBr/LiCoO 2是使用延展性反钙钛矿结构的 Li 2 OHBr 通过室温致密化制备的。研究了 Li 2 OHBr 的阳极分解对 LiCoO 2 /Li 2 OHBr 界面电阻和充放电稳定性的影响。Li 2 OHBr 在 LiCoO 2上在 3.6 V vs. Li/Li +下分解,并且在第一个循环中观察到不可逆的充电容量。这种阳极分解不会增加 LiCoO 2 /Li 2 OHBr 界面和 Li 2中的电阻OHBr。透射电子显微镜分析显示 Li 2 OHBr的阳极分解区域在 LiCoO 2和 Li 2 OHBr 相之间具有约 30 nm 的厚度。该厚度几乎与假设 Li 2 OHBr的单电子到双电子阳极分解反应的预期值一致。所得固体电解质界面将具有可接受的 Li +电导率并改善界面的粘附性。无定形磷酸锂的表面涂层可能通过抑制质子插入 LiCoO 2来稳定充放电反应。

































 京公网安备 11010802027423号
京公网安备 11010802027423号